Dysregulation of Metabolic Peptides Precedes Hyperinsulinemia and Inflammation Following Exposure to Rotenone in Rats
- PMID: 39851552
- PMCID: PMC11764466
- DOI: 10.3390/cells14020124
Dysregulation of Metabolic Peptides Precedes Hyperinsulinemia and Inflammation Following Exposure to Rotenone in Rats
Abstract
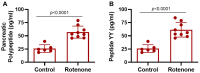
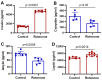
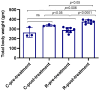
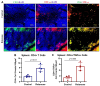
Rotenone, a naturally occurring compound derived from the roots of tropical plants, is used as a broad-spectrum insecticide, piscicide, and pesticide. It is a classical, high-affinity mitochondrial complex I inhibitor that causes not only oxidative stress, α-synuclein phosphorylation, DJ-1 (Parkinson's disease protein 7) modifications, and inhibition of the ubiquitin-proteasome system but it is also widely considered an environmental contributor to Parkinson's disease (PD). While prodromal symptoms, such as loss of smell, constipation, sleep disorder, anxiety/depression, and the loss of dopaminergic neurons in the substantia nigra of rotenone-treated animals, have been reported, alterations of metabolic hormones and hyperinsulinemia remain largely unknown and need to be investigated. Whether rotenone and its effect on metabolic peptides could be utilized as a biomarker for its toxic metabolic effects, which can cause long-term detrimental effects and ultimately lead to obesity, hyperinsulinemia, inflammation, and possibly gut-brain axis dysfunction, remains unclear. Here, we show that rotenone disrupts metabolic homeostasis, altering hormonal peptides and promoting infiltration of inflammatory T cells. Specifically, our results indicate a significant decrease in glucagon-like peptide-1 (GLP-1), C-peptide, and amylin. Interestingly, levels of several hormonal peptides related to hyperinsulinemia, such as insulin, leptin, pancreatic peptide (PP), peptide YY (PYY), and gastric inhibitory polypeptide (GIP), were significantly upregulated. Administration of rotenone to rats also increased body weight and activated macrophages and inflammatory T cells. These data strongly suggest that rotenone disrupts metabolic homeostasis, leading to obesity and hyperinsulinemia. The potential implications of these findings are vast, given that monitoring these markers in the blood could not only provide a crucial tool for assessing the extent of exposure and its relevance to obesity and inflammation but could also open new avenues for future research and potential therapeutic strategies.
Keywords: diabetes; hormonal peptides; hyperinsulinemia; inflammation; obesity; rotenone.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures




References
-
- White R.F., Steele L., O’Callaghan J.P., Sullivan K., Binns J.H., Golomb B.A., Bloom F.E., Bunker J.A., Crawford F., Graves J.C., et al. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex. 2016;74:449–475. doi: 10.1016/j.cortex.2015.08.022. - DOI - PMC - PubMed
-
- Betarbet, Greenamyre J.T. Chapter 14—Complex I Inhibition, Rotenone and Parkinson’s Disease. In: Nass R., Przedborski S., editors. Parkinson’s Disease Molecular and Therapeutic Insights from Model Systems. Volume 12 Academic Press; Cambridge, MA, USA: 2008.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

