A Real-World Comparison Between Adjuvant Docetaxel with Cyclophosphamide (TC) and Anthracycline-Taxane Chemotherapy in Early HER-2 Negative Breast Cancer
- PMID: 39851922
- PMCID: PMC11764166
- DOI: 10.3390/curroncol32010006
A Real-World Comparison Between Adjuvant Docetaxel with Cyclophosphamide (TC) and Anthracycline-Taxane Chemotherapy in Early HER-2 Negative Breast Cancer
Abstract
Background: Anthracycline-taxane chemotherapy is the gold standard in high-risk breast cancer (BC), despite the potential risk of congestive heart failure (CHF). A suitable alternative for anthracycline-sparing chemotherapy is through the combination of docetaxel and cyclophosphamide (TC).
Methods: Through a retrospective study of stage I-III HER2-negative BC, using administrative databases, we analyzed a total of 10,634 women treated with adjuvant chemotherapy in Ontario, Canada, between 2009 and 2017. We compared TC versus standardized anthracycline-taxane chemotherapies (ACT and FEC-D). We investigated the overall survival (OS), and explored the incidence of CHF, emergency department (ED) visits and febrile neutropenia.
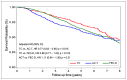
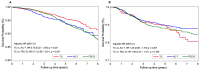
Results: With a median follow-up of 5.5 years, the 5-year analysis showed an increased OS in patients treated with TC, versus those treated with ACT, HR 0.77 (0.63-0.95, p = 0.015). Among ER+ BC, there was an increased OS in patients treated with ACT and FEC-D, versus those treated with TC, HR 0.70 (0.52-0.95, p = 0.021) and HR 0.71 (0.56-0.91, p = 0.007), respectively. There were no substantial differences in CHF, between TC and anthracycline-based treatments. Patients treated with TC and FEC-D had more ED visits, compared to those treated with ACT.
Conclusion: Our study shows that anthracycline-taxane regimens were the most commonly prescribed adjuvant chemotherapy options in HER2-negative BC. Women who received ACT had the lowest OS, likely due to their unfavorable pathology.
Keywords: adjuvant; doxorubicin; endocrine (hormone) receptor; epirubicin; human epidermal growth factor receptor 2 negative; invasive breast carcinoma; paclitaxel.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Dose-dense FEC followed by docetaxel versus docetaxel plus cyclophosphamide as adjuvant chemotherapy in women with HER2-negative, axillary lymph node-positive early breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG).Ann Oncol. 2016 Oct;27(10):1873-8. doi: 10.1093/annonc/mdw274. Epub 2016 Aug 8. Ann Oncol. 2016. PMID: 27502729 Clinical Trial.
-
Anthracycline and taxane-based chemotherapy versus docetaxel and cyclophosphamide in the adjuvant treatment of HER2-negative breast cancer patients: a systematic review and meta-analysis of randomized controlled trials.Breast Cancer Res Treat. 2019 Feb;174(1):27-37. doi: 10.1007/s10549-018-5055-9. Epub 2018 Nov 21. Breast Cancer Res Treat. 2019. PMID: 30465156
-
A Retrospective Analysis of Metronomic Cyclophosphamide, Methotrexate, and Fluorouracil (CMF) Versus Docetaxel and Cyclophosphamide (TC) as Adjuvant Treatment in Early Stage, Hormone Receptor Positive, HER2 Negative Breast Cancer.Clin Breast Cancer. 2022 Apr;22(3):e310-e318. doi: 10.1016/j.clbc.2021.09.007. Epub 2021 Sep 22. Clin Breast Cancer. 2022. PMID: 34753632
-
Anthracycline could be essential for triple-negative breast cancer: A randomised phase II study by the Kanagawa Breast Oncology Group (KBOG) 1101.Breast. 2019 Oct;47:1-9. doi: 10.1016/j.breast.2019.06.003. Epub 2019 Jun 15. Breast. 2019. PMID: 31229857 Clinical Trial.
-
Selection of adjuvant chemotherapy for treatment of node-positive breast cancer.Lancet Oncol. 2005 Nov;6(11):886-98. doi: 10.1016/S1470-2045(05)70424-1. Lancet Oncol. 2005. PMID: 16257797 Review.
References
-
- Female Breast Cancer—Cancer Stat Facts. [(accessed on 18 July 2023)]; Available online: https://seer.cancer.gov/statfacts/html/breast.html.
-
- Mayer E.L., Fesl C., Hlauschek D., Garcia-Estevez L., Burstein H.J., Zdenkowski N., Wette V., Miller K.D., Balic M., Mayer I.A., et al. Treatment Exposure and Discontinuation in the PALbociclib CoLlaborative Adjuvant Study of Palbociclib with Adjuvant Endocrine Therapy for Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Early Breast Cancer (PALLAS/AFT-05/ABCSG-42/BIG-14-03) J. Clin. Oncol. 2022;40:449–458. doi: 10.1200/JCO.21.01918. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

