Real-World Efficacy and Safety of Avelumab Plus Axitinib in Metastatic Renal Cell Carcinoma: Results from the Ambispective RAVE-Renal Study
- PMID: 39851927
- PMCID: PMC11764420
- DOI: 10.3390/curroncol32010011
Real-World Efficacy and Safety of Avelumab Plus Axitinib in Metastatic Renal Cell Carcinoma: Results from the Ambispective RAVE-Renal Study
Abstract
Background: The RAVE-Renal study was conducted to evaluate the real-world efficacy and safety of avelumab plus axitinib as a first-line therapy for patients with metastatic renal cell carcinoma (mRCC).
Methods: RAVE-Renal was a multicenter, noninterventional, ambispective study with both retrospective and prospective components. The study included adult patients with histologically confirmed mRCC, measurable disease per RECIST version 1.1, and no prior systemic therapy. Patients received avelumab (800 mg intravenously every 2 weeks) plus axitinib (5 mg orally twice daily). The primary endpoints were median progression-free survival (PFS) and objective response rate (ORR). The secondary endpoints included median OS, 1-year overall survival (OS) rate, and safety.
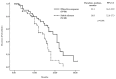
Results: A total of 125 patients from 13 sites were enrolled, with a median follow-up of 16.1 months. The median age was 61.0 years. The study population comprised 35.3% favorable, 49% intermediate, and 15.7% poor IMDC risk patients. The median PFS was 14.9 months (95% CI, 11.72-19.08). The ORR was 44.3% (95% CI, 32.5-56.1). The clinical benefit rate was 93.4%. The 1-year OS rate was 71.2%, with the median OS not reached. Any-grade treatment-related adverse events (TRAEs) occurred in 99 (79.2%) cases, including grade ≥3 TRAEs in 24 (19.2%).
Conclusions: Avelumab in combination with axitinib showed clinical benefits in a real-world setting, consistent with findings from a pivotal trial. The regimen was effective and well tolerated across various patient subgroups.
Keywords: RAVE-Renal ambispective study; avelumab plus axitinib; first-line therapy; metastatic renal cell carcinoma.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Tsimafeyeu I., Rahib L. The future landscape of cancer incidence and mortality until 2036 in the Russian Federation. J. Clin. Oncol. 2022;40((Suppl. 16)):e22518. doi: 10.1200/JCO.2022.40.16_suppl.e22518. - DOI
-
- Tsimafeyeu I. Nivolumab: 5 Years Since FDA Approval of the First Checkpoint Inhibitor for Renal Cell Carcinoma. Kidney Cancer. 2021;5:63–71. doi: 10.3233/KCA-200109. - DOI
-
- Tomita Y., Motzer R.J., Choueiri T.K., Rini B., Miyake H., Oya M., Albiges L., Aizawa M., Umeyama Y., Wang J., et al. Efficacy of avelumab plus axitinib versus sunitinib by numbers of IMDC risk factors and target tumor sites at baseline in advanced renal cell carcinoma: Long-term follow-up results from JAVELIN Renal 101. ESMO Open. 2023;8:102034. doi: 10.1016/j.esmoop.2023.102034. - DOI - PMC - PubMed
-
- FDA. [(accessed on 10 November 2024)]; Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-appro....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical