Trends in Real-World Clinical Outcomes of Patients with Anaplastic Lymphoma Kinase (ALK) Rearranged Non-Small Cell Lung Cancer (NSCLC) Receiving One or More ALK Tyrosine Kinase Inhibitors (TKIs): A Cohort Study in Ontario, Canada
- PMID: 39851929
- PMCID: PMC11764221
- DOI: 10.3390/curroncol32010013
Trends in Real-World Clinical Outcomes of Patients with Anaplastic Lymphoma Kinase (ALK) Rearranged Non-Small Cell Lung Cancer (NSCLC) Receiving One or More ALK Tyrosine Kinase Inhibitors (TKIs): A Cohort Study in Ontario, Canada
Abstract
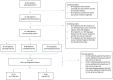
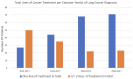
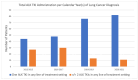
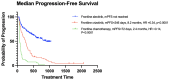
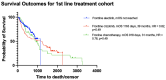
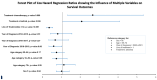
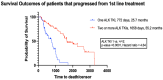
The treatment landscape for patients with advanced ALK-positive NSCLC has rapidly evolved following the approval of several ALK TKIs in Canada. However, public funding of ALK TKIs is mostly limited to the first line treatment setting. Using linked provincial health administrative databases, we examined real-world outcomes of patients with advanced ALK-positive NSCLC receiving ALK TKIs in Ontario between 1 January 2012 and 31 December 2021. Demographic, clinical characteristics and treatment patterns were summarized using descriptive statistics. Kaplan-Meier analysis was performed to evaluate progression-free survival (PFS) and overall survival (OS) among the treatment groups. A total of 413 patients were identified. Patients were administered alectinib (n = 154), crizotinib (n = 80), or palliative-intent chemotherapy (n = 55) in the first-line treatment. There was a significant difference in first-line PFS between the treatment groups. The median PFS (mPFS) was not reached for alectinib (95% CI, 568 days-not reached), compared to 8.2 months (95% CI, 171-294 days) for crizotinib (HR = 0.34, p < 0.0001) and 2.4 months (95% CI, 65-100 days) for chemotherapy (HR = 0.14, p < 0.0001). There was no significant difference in first-line OS between the treatment groups. In patients who received more than one line of treatment, there was a significant difference in mOS between patients who received two or more lines of ALK TKIs compared to those who received one line of ALK TKI (mOS = 55 months (95% CI, 400-987 days) and 26 months (95% CI, 1448-2644 days), respectively, HR = 4.64, p < 0.0001). This study confirms the effectiveness of ALK TKIs in real-world practice and supports the potential benefit of multiple lines of ALK TKI on overall survival in patients with ALK-positive NSCLC.
Keywords: ALK TKIs; ALK inhibitors; NSCLC; Ontario Canada; alectinib; crizotinib; first line treatment; lung cancer; real-world outcomes; sequential treatment.
Conflict of interest statement
LC is an employee of Hoffmann La-Roche, a pharmaceutical and diagnostics company. This retrospective study was conducted independent of LC’s place of employment. NBL has received institutional research funding from AZ, Eli Lilly, Neogenomics, Merck, Pfizer, BMS and GSK and travel funding for CME lectures from AZ, Janssen, MSD, Sanofi, Guardant Health and Roche. MT received consulting fees from CDA and Health Canada. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Cancer, Canadian Cancer Society. Canadienne du Lung and Bronchus Cancer Statistics. [(accessed on 17 December 2024)]. Available online: https://cancer.ca/en/cancer-information/cancer-types/lung/statistics.
-
- Williams A.S., Greer W., Bethune D., Craddock K.J., Flowerdew G., Xu Z. ALK+ Lung Adenocarcinoma in Never Smokers and Long-Term Ex-Smokers: Prevalence and Detection by Immunohistochemistry and Fluorescence in Situ Hybridization. Virchows Arch. 2016;469:533–540. doi: 10.1007/s00428-016-2005-y. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

