Retrospective, Non-Interventional, Multicenter Study on the Effectiveness and Safety of Intravesical Bacillus Calmette-Guerin in Patients with Non-Muscle-Invasive Bladder Cancer: Real-World Experience from Six Hospital Centers in Greece
- PMID: 39851934
- PMCID: PMC11763471
- DOI: 10.3390/curroncol32010018
Retrospective, Non-Interventional, Multicenter Study on the Effectiveness and Safety of Intravesical Bacillus Calmette-Guerin in Patients with Non-Muscle-Invasive Bladder Cancer: Real-World Experience from Six Hospital Centers in Greece
Abstract
Background: While the clinical application of SII-ONCO-Bacillus Calmette-Guerin (BCG) for non-muscle-invasive bladder cancer (NMIBC) is well established in Greece, there is a lack of real-world data on its effectiveness and safety. This retrospective, observational, multicenter, chart-review study aims to provide real-life data on the effectiveness and safety of SII-ONCO-BCG in patients with intermediate- and high-risk NMIBC.
Methods: From January 2016 to December 2023, medical records from six hospital centers were reviewed for adult patients with histologically confirmed stage Ta or T1 NMIBC (with or without carcinoma in situ [CIS]) who received at least one maintenance course of SII-ONCO-BCG after induction. Tumor recurrence and progression were monitored at scheduled time intervals. Primary outcomes included recurrence-free survival (RFS) and progression-free survival (PFS), while adverse events (AEs) constituted secondary outcomes.
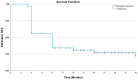
Results: A total of 162 patients receiving SII-ONCO-BCG were enrolled. Among all patients, 145 (89.5%) patients were men, 88 (54.3%) aged 70 years or older, 103 (63.6%) had T1, 43 (26.5%) Ta, and 21 (12.9%) concurrent CIS. The median follow-up duration was 28.9 months (range, 5-36) and the mean BCG intravesical instillation courses were 13.7 (range, 9-27). After 3-, 2-, and 1-year follow-up, RFS rates of 85.2% (95% CI, 79.7-90.7%), 85.8% (80.4-91.2%), and 87.0% (81.8-92.3%) were observed, respectively. The corresponding 3-, 2-, and 1-year PFS rates were 96.9% (94.2-99.6%), 96.9% (94.2-99.6%), and 97.5% (95.1-99.9%), respectively. During the whole follow-up period, 24 (14.8%) patients experienced at least one AE.
Conclusions: This real-world study demonstrates that SII-ONCO-BCG is an effective and safe treatment for patients with intermediate- and high-risk NMIBC.
Keywords: Bacillus Calmette–Guerin; Greece; multicenter study; non-muscle-invasive bladder.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Braasch M.R., Böhle A., O’Donnell M.A. Intravesical Instillation Treatment of Non–Muscle-Invasive Bladder Cancer. Eur. Urol. Suppl. 2009;8:549–555. doi: 10.1016/j.eursup.2009.06.009. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical