Columnar Mesophases and Organogels Formed by H-Bound Dimers Based on 3,6-Terminally Difunctionalized Triphenylenes
- PMID: 39851980
- PMCID: PMC11764606
- DOI: 10.3390/gels11010009
Columnar Mesophases and Organogels Formed by H-Bound Dimers Based on 3,6-Terminally Difunctionalized Triphenylenes
Abstract
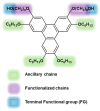
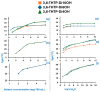
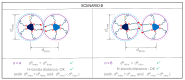
A series of triphenylene (TP) compounds-denoted 3,6-THTP-DiCnOH-bearing four hexyloxy ancillary chains and two variable-length alkoxy chains terminally functionalized with hydroxyl groups have been synthesized and characterized. The shorter homologs revealed mesogenic characteristics, giving rise to thermotropic mesophases in which π-stacked columns of H-bound dimers self-organize yielding superstructures. Molecular-scale models are proposed to account for their structural features. The three studied compounds yielded supramolecular gels in methanol; their ability to gelify higher alcohols was found to be enhanced by the presence of water. The intermediate homolog also gelled n-hexane. Compared to their isomeric 2,7-THTP-DiCnOH analogs, the 3,6-derivatives showed a higher tendency to give rise to LC phases (wider thermal ranges) and a lower organogelling ability (variety of gelled solvents, lower gels stabilities). The overall results are analyzed in terms of different kinds of competing H-bonds: intramolecular, face-to-face dimeric, lateral polymeric, and solvent-TP interactions.
Keywords: H-bonded dimers; columnar liquid crystals; organogel; triphenylene functionalized triphenylenes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures












References
-
- Goodby J.W., Collings P.J., Kato T., Tschierske C., Gleeson H.F., Raynes P., editors. Handbook of Liquid Crystals. 2nd ed. Wiley-VCH Verlag GmbH & Co.; Hoboken, NJ, USA: 2014. - DOI
-
- Kubo K., Mori A., Ujie S., Tschierske C. Synthesis and Properties of Columnar Liquid Crystals and Organogelators with a Bitropone Core. J. Oleo Sci. 2004;53:575–579. doi: 10.5650/jos.53.575. - DOI
-
- Kuang G.-C., Jia X.-R., Teng M.-J., Chen E.-Q., Li W.-S., Ji Y. Organogels and Liquid Crystalline Properties of Amino Acid-Based Dendrons: A Systematic Study on Structure-Property Relationship. Chem. Mater. 2011;24:71–80. doi: 10.1021/cm201913p. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

