Polymer Gels Based on PAMAM Dendrimers Functionalized with Caffeic Acid for Wound-Healing Applications
- PMID: 39852007
- PMCID: PMC11764813
- DOI: 10.3390/gels11010036
Polymer Gels Based on PAMAM Dendrimers Functionalized with Caffeic Acid for Wound-Healing Applications
Abstract
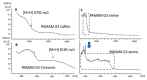
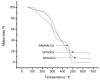
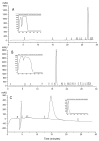
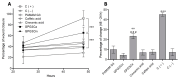
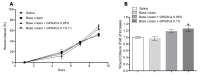
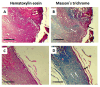
The wound-healing process has usually been related to therapeutic agents with antioxidant properties. Among them, caffeic acid, a cinnamic acid derivative, stands out. However, the use of this natural product is affected by its bioavailability and half-life. Nowadays, different approaches are being taken to improve the above-mentioned characteristics, as many active surface groups are present in polyamidoamine (PAMAM) dendrimers; without the need for extra cross-linking agents, physical gels are created by interactions such as hydrogen bonds, van der Waals forces, or π-π interactions based on the modification of the surface. One of these is functionalization with dendrimers, such as the poly(amidoamine) (PAMAM) family. To evaluate the effectiveness of functionalizing caffeic acid with PAMAM dendrimers, the in vitro and in vivo wound-healing properties of gel-PAMAM G3 conjugated with caffeic acid (GPG3Ca) and its precursor, cinnamic acid (GPG3Cin), were studied. The results showed no cytotoxicity and wound-healing activity at a concentration of 20 μg/mL in HaCaT cells with the GPG3Ca. Additionally, the ability to activate molecular mediators of the healing process was evidenced. Furthermore, GPG3Ca potentiated the in vivo wound-healing process. The positive effects and lack of cytotoxicity at the used concentration of the synthesized GPG3Ca on the wound-healing process could position it as an effective agent for wound-healing treatment.
Keywords: assay in vitro; caffeic and cinnamic acid; gel-PAMAM dendrimer; synthesis polymers gels; wound healing.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

