Kombucha Versus Vegetal Cellulose for Affordable Mucoadhesive (nano)Formulations
- PMID: 39852008
- PMCID: PMC11765165
- DOI: 10.3390/gels11010037
Kombucha Versus Vegetal Cellulose for Affordable Mucoadhesive (nano)Formulations
Abstract
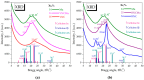
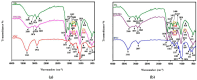
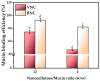
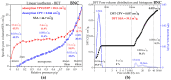
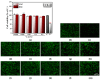
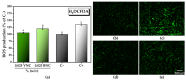
Cellulose nanofibers gained increasing interest in the production of medical devices such as mucoadhesive nanohydrogels due to their ability to retain moisture (high hydrophilicity), flexibility, superior porosity and durability, biodegradability, non-toxicity, and biocompatibility. In this work, we aimed to compare the suitability of selected bacterial and vegetal nanocellulose to form hydrogels for biomedical applications. The vegetal and bacterial cellulose nanofibers were synthesized from brewer's spent grains (BSG) and kombucha membranes, respectively. Two hydrogels were prepared, one based on the vegetal and the other based on the bacterial cellulose nanofibers (VNC and BNC, respectively). VNC was less opaque and more fluid than BNC. The cytocompatibility and in vitro antioxidant activity of the nanocellulose-based hydrogels were investigated using human gingival fibroblasts (HGF-1, ATCC CRL-2014). The investigation of the hydrogel-mucin interaction revealed that the BNC hydrogel had an approx. 2× higher mucin binding efficiency than the VNC hydrogel at a hydrogel/mucin ratio (mg/mg) = 4. The BNC hydrogel exhibited the highest potential to increase the number of metabolically active viable cells (107.60 ± 0.98% of cytotoxicity negative control) among all culture conditions. VNC reduced the amount of reactive oxygen species (ROS) by about 23% (105.5 ± 2.2% of C-) in comparison with the positive control, whereas the ROS level was slightly higher (120.2 ± 3.9% of C-) following the BNC hydrogel treatment. Neither of the two hydrogels showed antibacterial activity when assessed by the diffusion method. The data suggest that the BNC hydrogel based on nanocellulose from kombucha fermentation could be a better candidate for cytocompatible and mucoadhesive nanoformulations than the VNC hydrogel based on nanocellulose from brewer's spent grains. The antioxidant and antibacterial activity of BNC and both BNC and VNC, respectively, should be improved.
Keywords: brewer’s spent grains; cytocompatibility; gingival fibroblasts; hydrogels; kombucha fermentation; mucoadhesion; nanocellulose.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








Similar articles
-
Bioactive Hydrogel Formulation Based on Ferulic Acid-Grafted Nano-Chitosan and Bacterial Nanocellulose Enriched with Selenium Nanoparticles from Kombucha Fermentation.J Funct Biomater. 2024 Jul 22;15(7):202. doi: 10.3390/jfb15070202. J Funct Biomater. 2024. PMID: 39057323 Free PMC article.
-
Using in situ nanocellulose-coating technology based on dynamic bacterial cultures for upgrading conventional biomedical materials and reinforcing nanocellulose hydrogels.Biotechnol Prog. 2016 Jul 8;32(4):1077-84. doi: 10.1002/btpr.2280. Epub 2016 May 4. Biotechnol Prog. 2016. PMID: 27088548
-
Biocompatibility evaluation of densified bacterial nanocellulose hydrogel as an implant material for auricular cartilage regeneration.Appl Microbiol Biotechnol. 2014 Sep;98(17):7423-35. doi: 10.1007/s00253-014-5819-z. Epub 2014 May 28. Appl Microbiol Biotechnol. 2014. PMID: 24866945
-
Engineering nanocellulose hydrogels for biomedical applications.Adv Colloid Interface Sci. 2019 May;267:47-61. doi: 10.1016/j.cis.2019.03.002. Epub 2019 Mar 8. Adv Colloid Interface Sci. 2019. PMID: 30884359 Review.
-
Electroconductive Nanocellulose, a Versatile Hydrogel Platform: From Preparation to Biomedical Engineering Applications.Adv Healthc Mater. 2025 Jan;14(3):e2403983. doi: 10.1002/adhm.202403983. Epub 2024 Dec 12. Adv Healthc Mater. 2025. PMID: 39668476 Review.
References
-
- Bird D., Ravindra N.M. Transdermal drug delivery and patches—An overview. Med. Devices Sens. 2020;3:e10069. doi: 10.1002/mds3.10069. - DOI
-
- Rath R., Tevatia S., Rath A., Behl A., Modgil V., Sharma N. Mucoadhesive Systems in Dentistry: A Review. Indian. J. Dent. Res. 2016;4:25–29. doi: 10.14419/ijdr.v4i2.6283. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

