Exploring Methacrylated Gellan Gum 3D Bioprinted Patches Loaded with Tannic Acid or L-Ascorbic Acid as Potential Platform for Wound Dressing Application
- PMID: 39852011
- PMCID: PMC11765158
- DOI: 10.3390/gels11010040
Exploring Methacrylated Gellan Gum 3D Bioprinted Patches Loaded with Tannic Acid or L-Ascorbic Acid as Potential Platform for Wound Dressing Application
Abstract
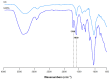
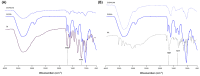
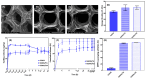
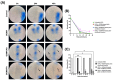
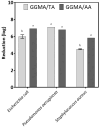
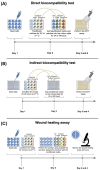
To improve wound healing, advanced biofabrication techniques are proposed here to develop customized wound patches to release bioactive agents targeting cell function in a controlled manner. Three-dimensional (3D) bioprinted "smart" patches, based on methacrylated gellan gum (GGMA), loaded with tannic acid (TA) or L-ascorbic acid (AA) have been manufactured. To improve stability and degradation time, gellan gum (GG) was chemically modified by grafting methacrylic moieties on the polysaccharide backbone. GGMA patches were characterized through physicochemical, morphological and mechanical evaluation. Kinetics release and antioxidant potential of TA and AA as well as antimicrobial activity against common pathogens Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli in accordance with ISO 22196:2011 are reported. The cytocompatibility of the patches was demonstrated by direct and indirect tests on human dermal fibroblasts (HDF) as per ISO 10993. The positive effect of GGMA patches on cell migration was assessed through a wound healing assay. The results highlighted that the patches are cytocompatible, speed up wound healing and can swell upon contact with the hydration medium and release TA and AA in a controlled way. Overall, the TA- and AA-loaded GGMA patches demonstrated suitable mechanical features; no cytotoxicity; and antioxidant, antimicrobial and wound healing properties, showing satisfactory potential for wound dressing applications.
Keywords: 3D bioprinting; L-ascorbic acid; methacrylated gellan gum patches; tannic acid; wound healing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Bioactive Composite Methacrylated Gellan Gum for 3D-Printed Bone Tissue-Engineered Scaffolds.Nanomaterials (Basel). 2023 Feb 19;13(4):772. doi: 10.3390/nano13040772. Nanomaterials (Basel). 2023. PMID: 36839140 Free PMC article.
-
Tannic acid-reinforced methacrylated chitosan/methacrylated silk fibroin hydrogels with multifunctionality for accelerating wound healing.Carbohydr Polym. 2020 Nov 1;247:116689. doi: 10.1016/j.carbpol.2020.116689. Epub 2020 Jun 25. Carbohydr Polym. 2020. PMID: 32829817
-
Ascorbic acid-loaded gellan-g-poly(ethylene glycol) methacrylate matrix as a wound-healing material.Int J Biol Macromol. 2023 Nov 1;251:126243. doi: 10.1016/j.ijbiomac.2023.126243. Epub 2023 Aug 13. Int J Biol Macromol. 2023. PMID: 37582430
-
Exploring the Antioxidant Potential of Gellan and Guar Gums in Wound Healing.Pharmaceutics. 2023 Aug 17;15(8):2152. doi: 10.3390/pharmaceutics15082152. Pharmaceutics. 2023. PMID: 37631366 Free PMC article. Review.
-
Gellan Gum in Wound Dressing Scaffolds.Polymers (Basel). 2022 Sep 30;14(19):4098. doi: 10.3390/polym14194098. Polymers (Basel). 2022. PMID: 36236046 Free PMC article. Review.
Cited by
-
Development and Characterization of Biocompatible Chitosan-Aloe Vera Films Functionalized with Gluconolactone and Sorbitol for Advanced Wound Healing Applications.ACS Appl Mater Interfaces. 2025 Mar 12;17(10):15196-15207. doi: 10.1021/acsami.5c00715. Epub 2025 Feb 25. ACS Appl Mater Interfaces. 2025. PMID: 39999379 Free PMC article.
-
Tannic Acid-Loaded Gellan Gum Hydrogels Reduce In Vitro Chemokine Expression in Oral Cells.Int J Mol Sci. 2025 Jun 11;26(12):5578. doi: 10.3390/ijms26125578. Int J Mol Sci. 2025. PMID: 40565042 Free PMC article.
References
-
- Wallace H.A., Basehore B.M., Zito P.M. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. Wound Healing Phases. - PubMed
-
- Ferroni L., Gardin C., D’Amora U., Calzà L., Ronca A., Tremoli E., Ambrosio L., Zavan B. Exosomes of Mesenchymal Stem Cells Delivered from Methacrylated Hyaluronic Acid Patch Improve the Regenerative Properties of Endothelial and Dermal Cells. Biomater. Adv. 2022;139:213000. doi: 10.1016/j.bioadv.2022.213000. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

