The Pre-Polarization and Concentration of Cells near Micro-Electrodes Using AC Electric Fields Enhances the Electrical Cell Lysis in a Sessile Drop
- PMID: 39852073
- PMCID: PMC11763957
- DOI: 10.3390/bios15010022
The Pre-Polarization and Concentration of Cells near Micro-Electrodes Using AC Electric Fields Enhances the Electrical Cell Lysis in a Sessile Drop
Abstract
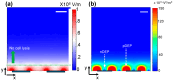
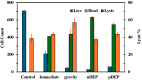
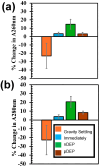
Cell lysis is the starting step of many biomedical assays. Electric field-based cell lysis is widely used in many applications, including point-of-care (POC) applications, because it provides an easy one-step solution. Many electric field-based lysis methods utilize micro-electrodes to apply short electric pulses across cells. Unfortunately, these cell lysis devices produce relatively low cell lysis efficiency as electric fields do not reach a significant portion of cells in the sample. Additionally, the utility of syringe pumps for flow cells in and out of the microfluidics channel causes cell loss and low throughput cell lysis. To address these critical issues, we suspended the cells in a sessile drop and concentrated on the electrodes. We used low-frequency AC electric fields (1 Vpp, 0-100 kHz) to drive the cells effectively towards electrodes and lysed using a short pulse of 10 V. A post-lysis analysis was performed using a hemocytometer, UV-vis spectroscopy, and fluorescence imaging. The results show that the pre-electric polarization of cells, prior to applying short electrical pulses, enhances the cell lysis efficiency. Additionally, the application of AC electric fields to concentrate cells on the electrodes reduces the assay time to about 4 min. In this study, we demonstrated that low-frequency AC electric fields can be used to pre-polarize and concentrate cells near micro-electrodes and improve cell lysis efficiency. Due to the simplicity and rapid cell lysis, this method may be suitable for POC assay development.
Keywords: cell lysis; dielectrophoresis and AC electroosmosis; electric fields; induced transmembrane potential.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

