Surface Plasmon Resonance-Based Biodetection Systems: Principles, Progress and Applications-A Comprehensive Review
- PMID: 39852086
- PMCID: PMC11763797
- DOI: 10.3390/bios15010035
Surface Plasmon Resonance-Based Biodetection Systems: Principles, Progress and Applications-A Comprehensive Review
Abstract
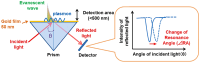
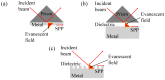
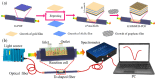
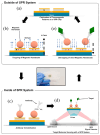
Surface Plasmon Resonance (SPR)-based biodetection systems have emerged as powerful tools for real-time, label-free biomolecular interaction analysis, revolutionizing fields such as diagnostics, drug discovery, and environmental monitoring. This review highlights the foundational principles of SPR, focusing on the interplay of evanescent waves and surface plasmons that underpin its high sensitivity and specificity. Recent advancements in SPR technology, including enhancements in sensor chip materials, integration with nanostructures, and coupling with complementary detection techniques, are discussed to showcase their role in improving analytical performance. The paper also explores diverse applications of SPR biodetection systems, ranging from pathogen detection and cancer biomarker identification to food safety monitoring and environmental toxin analysis. By providing a comprehensive overview of technological progress and emerging trends, this review underscores the transformative potential of SPR-based biodetection systems in addressing critical scientific and societal challenges. Future directions and challenges, including miniaturization, cost reduction, and expanding multiplexing capabilities, are also presented to guide ongoing research and development in this rapidly evolving field.
Keywords: Surface Plasmon Resonance; biodetection systems; drug delivery; environmental monitoring; food safety; plasmonics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



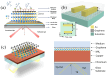
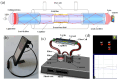
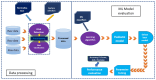
References
-
- Divya J., Selvendran S., Raja A.S., Sivasubramanian A. Surface Plasmon Based Plasmonic Sensors: A Review on Their Past, Present and Future. Biosens. Bioelectron. X. 2022;11:100175. doi: 10.1016/j.biosx.2022.100175. - DOI
-
- Butt M.A., Khonina S.N., Kazanskiy N.L. Plasmonics: A Necessity in the Field of Sensing-A Review (Invited) Fiber Integr. Opt. 2021;40:14–47. doi: 10.1080/01468030.2021.1902590. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

