Developing a Label-Free Infrared Spectroscopic Analysis with Chemometrics and Computational Enhancement for Assessing Lupus Nephritis Activity
- PMID: 39852090
- PMCID: PMC11763532
- DOI: 10.3390/bios15010039
Developing a Label-Free Infrared Spectroscopic Analysis with Chemometrics and Computational Enhancement for Assessing Lupus Nephritis Activity
Abstract
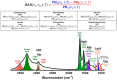
Patterns of disease and therapeutic responses vary widely among patients with autoimmune glomerulonephritis. This study introduces groundbreaking personalized infrared (IR)-based diagnostics for real-time monitoring of disease status and treatment responses in lupus nephritis (LN). We have established a relative absorption difference (RAD) equation to assess characteristic spectral indices based on the temporal peak heights (PHs) of two characteristic serum absorption bands: ν1 as the target signal and ν2 as the PH reference for the ν1 absorption band, measured at each dehydration time (t) during dehydration. The RAD gap (Ψ), defined as the difference in the RAD values between the initial and final stages of serum dehydration, enables the measurement of serum levels of IgG glycosylation (ν1 (1030 cm-1), ν2 (1171 cm-1)), serum lactate (ν1 (1021 cm-1), ν2 (1171 cm-1)), serum hydrophobicity (ν1 (2930 cm-1), ν2 (2960 cm-1)), serum hydrophilicity (ν1 (1550 cm-1), ν2 (1650 cm-1)), and albumin (ν1 (1400 cm-1), ν2 (1450 cm-1)). Furthermore, this IR-based assay incorporates an innovative algorithm and our proprietary iPath software (ver. 1.0), which calculates the prognosis prediction function (PPF, Φ) from the RAD gaps of five spectral markers and correlates these with conventional clinical renal biomarkers. We propose that this algorithm-assisted, IR-based approach can augment the patient-centric care of LN patients, particularly by focusing on changes in serum IgG glycosylation.
Keywords: FTIR spectroscopy; IgG glycosylation; iPath; lupus nephritis; prognosis prediction function; relative absorption difference.
Conflict of interest statement
FWKT has received research project grants from AstraZeneca Limited, OncoOne, Rigel Pharmaceuticals, and Thornton and Ross Ltd and has consultancy agreements with OncoOne, Rigel Pharmaceuticals, Retrophin, Travere Therapeutics. The other authors declare no conflicts of interest.
Figures








References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

