Mapping Surface Potential in DNA Aptamer-Neurochemical and Membrane-Ion Interactions on the SOS Substrate Using Terahertz Microscopy
- PMID: 39852097
- PMCID: PMC11764386
- DOI: 10.3390/bios15010046
Mapping Surface Potential in DNA Aptamer-Neurochemical and Membrane-Ion Interactions on the SOS Substrate Using Terahertz Microscopy
Abstract
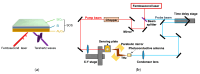
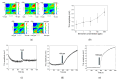
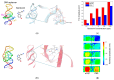
In this study, we utilized a terahertz chemical microscope (TCM) to map surface potential changes induced by molecular interactions on silicon-on-sapphire (SOS) substrates. By functionalizing the SOS substrate with DNA aptamers and an ion-selective membrane, we successfully detected and visualized aptamer-neurochemical complexes through the terahertz amplitude. Additionally, comparative studies of DNA aptamers in PBS buffer and artificial cerebrospinal fluid (aCSF) were performed by computational structure modeling and terahertz measurements. Beyond neurochemicals, we also investigated calcium ions, measuring their concentrations in PDMS-fabricated micro-wells using minimal sample volumes. Our results highlight the capability of TCM as a powerful, label-free, and sensitive platform for the probing and mapping of surface potential arising from molecular interactions, with broad implications for biomedical diagnostics and research.
Keywords: DNA aptamer–neurochemical complexes; SOS substrate; artificial cerebrospinal fluid; membrane–ion interactions; surface potential; terahertz chemical microscope.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Multifunctional terahertz microscopy for biochemical and chemical imaging and sensing.Biosens Bioelectron. 2023 Jan 15;220:114901. doi: 10.1016/j.bios.2022.114901. Epub 2022 Nov 17. Biosens Bioelectron. 2023. PMID: 36410157
-
High-sensitivity small-molecule detection of microcystin-LR cyano-toxin using a terahertz-aptamer biosensor.Analyst. 2021 Dec 6;146(24):7583-7592. doi: 10.1039/d1an01577j. Analyst. 2021. PMID: 34780591
-
Label-Free Measurement of CD63 Positive Extracellular Vesicles Using Terahertz Chemical Microscopy.ACS Sens. 2024 Jun 28;9(6):3244-3252. doi: 10.1021/acssensors.4c00590. Epub 2024 May 24. ACS Sens. 2024. PMID: 38785322
-
Aptamer-modified biosensors to visualize neurotransmitter flux.J Neurosci Methods. 2022 Jan 1;365:109386. doi: 10.1016/j.jneumeth.2021.109386. Epub 2021 Oct 13. J Neurosci Methods. 2022. PMID: 34653500 Review.
-
Aptamer Renaissance for Neurochemical Biosensing.ACS Nano. 2024 Jan 30;18(4):2552-2563. doi: 10.1021/acsnano.3c09576. Epub 2024 Jan 18. ACS Nano. 2024. PMID: 38236046 Free PMC article. Review.
References
-
- Lee H., Lee W., Lee J.H., Yoon D.S. Surface potential analysis of nanoscale biomaterials and devices using Kelvin probe force microscopy. J. Nanomater. 2016;2016:4209130. doi: 10.1155/2016/4209130. - DOI
-
- Brown M.A., Abbas Z., Kleibert A., Green R.G., Goel A., May S., Squires T.M. Determination of surface potential and electrical double-layer structure at the aqueous electrolyte-nanoparticle interface. Phys. Rev. X. 2016;6:011007. doi: 10.1103/PhysRevX.6.011007. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

