Impact of Thrombopoietin Receptor Agonists on Pathophysiology of Pediatric Immune Thrombocytopenia
- PMID: 39852180
- PMCID: PMC11763769
- DOI: 10.3390/cimb47010065
Impact of Thrombopoietin Receptor Agonists on Pathophysiology of Pediatric Immune Thrombocytopenia
Abstract
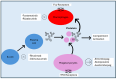
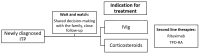
Immune thrombocytopenia (ITP) in pediatric patients is a common cause of isolated thrombocytopenia. Various pathophysiological mechanisms are implicated in ITP pathogenesis, including the production of autoantibodies against components of platelets (PLTs) by B-cells, the activation of the complement system, phagocytosis by macrophages mediated by Fcγ receptors, the dysregulation of T cells, and reduced bone marrow megakaryopoiesis. ITP is commonly manifested with skin and mucosal bleeding, and it is a diagnosis of exclusion. In some ITP cases, the disease is self-limiting, and treatment is not required, but chronic-persistent disease can also be developed. In these cases, anti-CD20 monoclonal antibodies, such as rituximab and thrombopoietin (TPO) receptor agonists, can be used. TPO agonists have become standard of care today. It has been reported in the published literature that the efficacy of TPO-RAs can be up to 80% in the achievement of several end goals, such as PLT counts. In the current literature review, the data regarding the impact of TPO agonists in the pathogenesis of ITP and treatment outcomes of the patients are examined. In the era of precision medicine, targeted and individualized therapies are crucial to achieving better outcomes for pediatric patients with ITP, especially when chronic refractory disease is developed.
Keywords: eltrombopag; immune thrombocytopenia; pediatrics; romiplostim; thrombopoietin receptor agonists.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Eltrombopag modulates the phenotypic evolution and potential immunomodulatory roles of monocytes/macrophages in immune thrombocytopenia.Platelets. 2023 Dec;34(1):2135694. doi: 10.1080/09537104.2022.2135694. Epub 2022 Oct 25. Platelets. 2023. PMID: 36281771
-
Treatment patterns and outcomes of second-line rituximab and thrombopoietin receptor agonists in adult immune thrombocytopenia: A Canadian retrospective cohort study.Thromb Res. 2022 Dec;220:5-11. doi: 10.1016/j.thromres.2022.09.021. Epub 2022 Sep 29. Thromb Res. 2022. PMID: 36257098
-
Thrombopoietin receptor agonists and rituximab for treatment of pediatric immune thrombocytopenia: A systematic review and meta-analysis of prospective clinical trials.Pediatr Blood Cancer. 2022 Mar;69(3):e29447. doi: 10.1002/pbc.29447. Epub 2021 Dec 28. Pediatr Blood Cancer. 2022. PMID: 34962697
-
Thrombopoietin Receptor Agonists in Children with Immune Thrombocytopenia: A New Therapeutic Era.Endocr Metab Immune Disord Drug Targets. 2021;21(3):397-406. doi: 10.2174/1871530320666200531142244. Endocr Metab Immune Disord Drug Targets. 2021. PMID: 32473624 Review.
-
Romiplostim as a treatment for immune thrombocytopenia: a review.J Blood Med. 2015 Jan 19;6:37-44. doi: 10.2147/JBM.S47240. eCollection 2015. J Blood Med. 2015. PMID: 25632241 Free PMC article. Review.
References
-
- Neunert C., Despotovic J., Haley K., Lambert M.P., Nottage K., Shimano K., Bennett C., Klaassen R., Stine K., Thompson A., et al. Thrombopoietin Receptor Agonist Use in Children: Data From the Pediatric ITP Consortium of North America ICON2 Study. Pediatr. Blood Cancer. 2016;63:1407–1413. doi: 10.1002/pbc.26003. - DOI - PMC - PubMed
-
- Wei P. Hematopoietic Growth Factors in Oncology. Springer; Berlin/Heidelberg, Germany: 2010. Thrombopoietin Factors; pp. 75–93.
Publication types
LinkOut - more resources
Full Text Sources