Osteopenia Metabolomic Biomarkers for Early Warning of Osteoporosis
- PMID: 39852408
- PMCID: PMC11767427
- DOI: 10.3390/metabo15010066
Osteopenia Metabolomic Biomarkers for Early Warning of Osteoporosis
Abstract
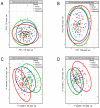
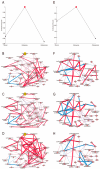
Introduction: This study aimed to capture the early metabolic changes before osteoporosis occurs and identify metabolomic biomarkers at the osteopenia stage for the early prevention of osteoporosis. Materials and Methods: Metabolomic data were generated from normal, osteopenia, and osteoporosis groups with 320 participants recruited from the Nicheng community in Shanghai. We conducted individual edge network analysis (iENA) combined with a random forest to detect metabolomic biomarkers for the early warning of osteoporosis. Weighted Gene Co-Expression Network Analysis (WGCNA) and mediation analysis were used to explore the clinical impacts of metabolomic biomarkers. Results: Visual separations of the metabolic profiles were observed between three bone mineral density (BMD) groups in both genders. According to the iENA approach, several metabolites had significant abundance and association changes in osteopenia participants, confirming that osteopenia is a critical stage in the development of osteoporosis. Metabolites were further selected to identify osteopenia (nine metabolites in females; eight metabolites in males), and their ability to discriminate osteopenia was improved significantly compared to traditional bone turnover markers (BTMs) (female AUC = 0.717, 95% CI 0.547-0.882, versus BTMs: p = 0.036; male AUC = 0.801, 95% CI 0.636-0.966, versus BTMs: p = 0.007). The roles of the identified key metabolites were involved in the association between total fat-free mass (TFFM) and osteopenia in females. Conclusion: Osteopenia was identified as a tipping point during the development of osteoporosis with metabolomic characteristics. A few metabolites were identified as candidate early-warning biomarkers by machine learning analysis, which could indicate bone loss and provide new prevention guidance for osteoporosis.
Keywords: biomarkers; bone mineral density; early warning; metabolomics; osteopenia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Discovery of potential biomarkers for osteoporosis using LC-MS/MS metabolomic methods.Osteoporos Int. 2019 Jul;30(7):1491-1499. doi: 10.1007/s00198-019-04892-0. Epub 2019 Feb 18. Osteoporos Int. 2019. PMID: 30778642
-
The diagnostic value of the combined application of blood lipid metabolism markers and interleukin-6 in osteoporosis and osteopenia.Lipids Health Dis. 2025 Feb 5;24(1):38. doi: 10.1186/s12944-025-02456-2. Lipids Health Dis. 2025. PMID: 39910539 Free PMC article.
-
Combining untargeted and targeted metabolomic profiling reveals principal differences between osteopenia, Osteoporosis and healthy controls.Aging Clin Exp Res. 2025 Jan 21;37(1):28. doi: 10.1007/s40520-024-02923-3. Aging Clin Exp Res. 2025. PMID: 39833609 Free PMC article.
-
Fracture risk and bone health in adrenal adenomas with mild autonomous cortisol secretion/subclinical hypercortisolism: a systematic review, meta-analysis and meta-regression.J Bone Miner Res. 2024 Aug 5;39(7):885-897. doi: 10.1093/jbmr/zjae067. J Bone Miner Res. 2024. PMID: 38703381
-
Effects of resistance training on microcirculation of bone tissue and bone turnover markers in postmenopausal women with osteopenia or osteoporosis: A systematic review.Clin Hemorheol Microcirc. 2025;89(1):161-174. doi: 10.3233/CH-249105. Clin Hemorheol Microcirc. 2025. PMID: 39911123
References
-
- Bliuc D., Alarkawi D., Nguyen T.V., Eisman J.A., Center J.R. Risk of subsequent fractures and mortality in elderly women and men with fragility fractures with and without osteoporotic bone density: The Dubbo Osteoporosis Epidemiology Study. J. Bone Miner. Res. 2015;30:637–646. doi: 10.1002/jbmr.2393. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

