Light Regulates Secreted Metabolite Production and Antagonistic Activity in Trichoderma
- PMID: 39852429
- PMCID: PMC11767173
- DOI: 10.3390/jof11010009
Light Regulates Secreted Metabolite Production and Antagonistic Activity in Trichoderma
Abstract
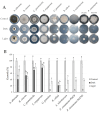
Secondary metabolism is one of the main mechanisms Trichoderma uses to explore and colonize new niches, and 6-pentyl-α-pyrone (6-PP) is an important secondary metabolite in this process. This work focused on standardizing a method to investigate the production of 6-PP. Ethanol and ethyl acetate were both effective solvents for quantifying 6-PP in solution and had limited solubility in potato-dextrose-broth media. The 6-PP extraction using ethyl acetate provided a rapid and efficient process to recover this metabolite. The 6-PP was readily produced during the development of Trichoderma atroviride growing in the dark, but light suppressed its production. The 6-PP was purified, and its spectrum by nuclear magnetic resonance and mass spectroscopy was identical to that of commercial 6-PP. Light also induced or suppressed other unidentified metabolites in several other species of Trichoderma. The antagonistic activity of T. atroviride was influenced by light, as suppression of plant pathogens was greater in the dark. The secreted metabolite production on potato-dextrose-agar was differentially regulated by light, indicating that Trichoderma produced several metabolites with antagonistic activity against plant pathogens. Light has an important influence on the secondary metabolism and antagonistic activity of Trichoderma, and this trait is of key relevance for selecting antagonistic Trichoderma strains for plant protection.
Keywords: antagonism; biocontrol; crop protection; fungal biotechnology; plant pathogen; secondary metabolism.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in this study’s design, the collection, analysis, or interpretation of data, the writing of this manuscript, or the decision to publish the results.
Figures







References
-
- Mukherjee P.K., Mendoza-Mendoza A., Zeilinger S., Horwitz B.A. Mycoparasitism as a mechanism of Trichoderma-mediated suppression of plant diseases. Fungal Biol. Rev. 2022;39:15–33. doi: 10.1016/j.fbr.2021.11.004. - DOI
-
- Kubicek C.P., Herrera-Estrella A., Seidl-Seiboth V., Martinez D.A., Druzhinina I.S., Thon M., Zeilinger S., Casas-Flores S., Horwitz B.A., Mukherjee P.K., et al. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol. 2011;12:R40. doi: 10.1186/gb-2011-12-4-r40. - DOI - PMC - PubMed
-
- Zeilinger S., Gruber S., Bansal R., Mukherjee P.K. Secondary metabolism in Trichoderma—Chemistry meets genomics. Fungal Biol. Rev. 2016;30:74–90. doi: 10.1016/j.fbr.2016.05.001. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

