A Low-Cost Electrochemical Cell Sensor Based on MWCNT-COOH/α-Fe2O3 for Toxicity Detection of Drinking Water Disinfection Byproducts
- PMID: 39852761
- PMCID: PMC11767749
- DOI: 10.3390/nano15020146
A Low-Cost Electrochemical Cell Sensor Based on MWCNT-COOH/α-Fe2O3 for Toxicity Detection of Drinking Water Disinfection Byproducts
Abstract
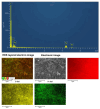
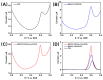
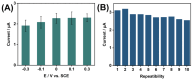
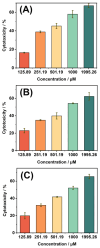
The disinfection of drinking water is essential for eliminating pathogens and preventing waterborne diseases. However, this process generates various disinfection byproducts (DBPs), which toxicological research indicates can have detrimental effects on living organisms. Moreover, the safety of these DBPs has not been sufficiently assessed, underscoring the need for a comprehensive evaluation of their toxic effects and associated health risks. Compared to traditional methods for studying the toxicity of pollutants, emerging electrochemical sensing technologies offer advantages such as simplicity, speed, and sensitivity, presenting an effective means for toxicity research on pollutants. However, challenges remain in this field, including the need to improve electrode sensitivity and reduce electrode costs. In this study, a pencil graphite electrode (PGE) was modified with carboxylated multi-walled carbon nanotubes (MWCNT-COOH) and nano-iron (III) oxide (α-Fe2O3) to fabricate a low-cost electrode with excellent electrocatalytic performance for cell-active substances. Subsequently, a novel cellular electrochemical sensor was constructed for the sensitive detection of the toxicity of three drinking water DBPs. The half inhibitory concentration (IC50) values of 2-chlorophenylacetonitrile (2-CPAN), 3-chlorophenylacetonitrile (3-CPAN), and 4-chlorophenylacetonitrile (4-CPAN) for HepG2 cells were 660.69, 831.76, and 812.83 µM, respectively. This study provides technical support and scientific evidence for the toxicity detection and safety assessment of emerging contaminants.
Keywords: biosensor; cytotoxicity; disinfection byproducts; multi-walled carbon nanotubes; nano-iron (III) oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Zhang Z., Zhu Q., Huang C., Yang M., Li J., Chen Y., Yang B., Zhao X. Comparative cytotoxicity of halogenated aromatic DBPs and implications of the corresponding developed QSAR model to toxicity mechanisms of those DBPs: Binding interactions between aromatic DBPs and catalase play an important role. Water Res. 2020;170:115283. doi: 10.1016/j.watres.2019.115283. - DOI - PubMed
-
- Jiang L., Luo J., Wei W., Song M., Shi W., Li A., Zhou Q., Pan Y. Comparative cytotoxicity analyses of disinfection byproducts in drinking water using dimensionless parameter scaling method: Effect of halogen substitution type and number. Water Res. 2023;240:120087. doi: 10.1016/j.watres.2023.120087. - DOI - PubMed
LinkOut - more resources
Full Text Sources

