Evaluation of Influenza Vaccine Effectiveness from 2021 to 2024: A Guangdong-Based Test-Negative Case-Control Study
- PMID: 39852783
- PMCID: PMC11768588
- DOI: 10.3390/vaccines13010004
Evaluation of Influenza Vaccine Effectiveness from 2021 to 2024: A Guangdong-Based Test-Negative Case-Control Study
Abstract
Background: The influenza virus's high mutation rate requires the annual reformulation and administration of the vaccine. Therefore, its vaccine effectiveness (VE) must be evaluated annually.
Aim: Estimate the effectiveness of the influenza vaccine and analyze the impact of age, seasonal variations, and the vaccination to sample collection interval on VE.
Methods: The study used a test-negative case-control (TNCC) design to collect data from patients under 18 years of age who presented with acute respiratory infection (ARI) symptoms and underwent influenza virus testing at a national children's regional medical center in Guangdong Province between October 2021 and January 2024, spanning three influenza seasons. VE was estimated using unconditional logistic regression.
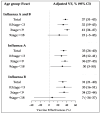
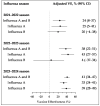
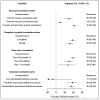
Results: A total of 27,670 patient data entries were analyzed. The VE against all influenza viruses across the three seasons was 37% (95% CI: 31-43), with the lowest VE of 24% (95% CI: 8-37) observed in the 2021-2022 season. In children aged 0.5 to <3 years, the VE was 32% (95% CI: 19-43). The effectiveness for samples collected at intervals of 0.5-2 months, 3-6 months, and over 6 months after vaccination was 39% (95% CI: 32-46), 30% (95% CI: 19-40), and 28% (95% CI: 5-46).
Conclusions: Across three influenza seasons, at least one-third of vaccinated individuals were protected from influenza in outpatient settings. Given that children are at high risk, improving vaccination management is recommended, and parents should be encouraged to vaccinate their children before each influenza season.
Keywords: China; case–control; influenza; test-negative; vaccination.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Early estimates of seasonal influenza vaccine effectiveness - United States, January 2015.MMWR Morb Mortal Wkly Rep. 2015 Jan 16;64(1):10-5. MMWR Morb Mortal Wkly Rep. 2015. PMID: 25590680 Free PMC article.
-
[Evaluation of the influenza vaccine effectiveness among children aged 6 to 72 months based on the test-negative case control study design].Zhonghua Yu Fang Yi Xue Za Zhi. 2019 Jun 6;53(6):576-580. doi: 10.3760/cma.j.issn.0253-9624.2019.06.007. Zhonghua Yu Fang Yi Xue Za Zhi. 2019. PMID: 31177753 Chinese.
-
Association of Prior Vaccination With Influenza Vaccine Effectiveness in Children Receiving Live Attenuated or Inactivated Vaccine.JAMA Netw Open. 2018 Oct 5;1(6):e183742. doi: 10.1001/jamanetworkopen.2018.3742. JAMA Netw Open. 2018. PMID: 30646262 Free PMC article.
-
Inactivated influenza vaccine effectiveness and an analysis of repeated vaccination for children during the 2016/17 season.Vaccine. 2018 Sep 5;36(37):5510-5518. doi: 10.1016/j.vaccine.2018.07.065. Epub 2018 Aug 6. Vaccine. 2018. PMID: 30093289
-
The impact of repeated vaccination on influenza vaccine effectiveness: a systematic review and meta-analysis.BMC Med. 2019 Jan 10;17(1):9. doi: 10.1186/s12916-018-1239-8. BMC Med. 2019. PMID: 30626399 Free PMC article.
References
-
- Bonilla F.A., Khan D.A., Ballas Z.K., Chinen J., Frank M.M., Hsu J.T., Keller M., Kobrynski L.J., Komarow H.D., Mazer B., et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J. Allergy Clin. Immunol. 2015;136:1186–1205.e78. doi: 10.1016/j.jaci.2015.04.049. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

