Distinct Neutralising and Complement-Fixing Antibody Responses Can Be Induced to the Same Antigen in Haemodialysis Patients After Immunisation with Different Vaccine Platforms
- PMID: 39852786
- PMCID: PMC11768972
- DOI: 10.3390/vaccines13010007
Distinct Neutralising and Complement-Fixing Antibody Responses Can Be Induced to the Same Antigen in Haemodialysis Patients After Immunisation with Different Vaccine Platforms
Abstract
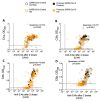
Background/Objectives: Generalised immune dysfunction in chronic kidney disease, especially in patients requiring haemodialysis (HD), significantly enhances the risk of severe infections. Vaccine-induced immunity is typically reduced in HD populations. The SARS-CoV-2 pandemic provided an opportunity to examine the magnitude and functionality of antibody responses in HD patients to a previously unencountered antigen-Spike (S)-glycoprotein-after vaccination with different vaccine platforms (viral vector (VV); mRNA (mRV)). Methods: We compared the total and functional anti-S antibody responses (cross-variant neutralisation and complement binding) in 187 HD patients and 43 healthy controls 21-28 days after serial immunisation. Results: After 2 doses of the same vaccine, HD patients had anti-S antibody levels and a complement binding capacity comparable to controls. However, 2 doses of mRV induced greater polyfunctional antibody responses than VV (defined by the presence of both complement binding and cross-variant neutralisation activity). Interestingly, an mRV boost after 2 doses of VV significantly enhanced antibody functionality in HD patients without a prior history of SARS-CoV-2 infection. Conclusions: HD patients can generate near-normal, functional antigen-specific antibody responses following serial vaccination to a novel antigen. Encouragingly, exploiting immunological memory by using mRNA vaccines and boosting may improve the success of vaccination strategies in this vulnerable patient population.
Keywords: SARS-CoV-2; antibody; haemodialysis; immune responses; vaccine.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Khan I.H., Catto G.R. Long-term complications of dialysis: Infection. Kidney Int. Suppl. 1993;41:S143–S148. - PubMed
-
- Schrauben S.J., Chen H.Y., Lin E., Jepson C., Yang W., Scialla J.J., Fischer M.J., Lash J.P., Fink J.C., Hamm L.L., et al. Hospitalizations among adults with chronic kidney disease in the United States: A cohort study. PLoS Med. 2020;17:e1003470. doi: 10.1371/journal.pmed.1003470. - DOI - PMC - PubMed
-
- Steenkamp R., Rao A., Fraser S. UK Renal Registry 18th Annual Report (December 2015) Chapter 5: Survival and Causes of Death in UK Adult Patients on Renal Replacement Therapy in 2014: National and Centre-specific Analyses. Nephron. 2016;132((Suppl. S1)):111–144. doi: 10.1159/000444819. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

