Regulating Immune Responses Induced by PEGylated Messenger RNA-Lipid Nanoparticle Vaccine
- PMID: 39852793
- PMCID: PMC11768904
- DOI: 10.3390/vaccines13010014
Regulating Immune Responses Induced by PEGylated Messenger RNA-Lipid Nanoparticle Vaccine
Abstract
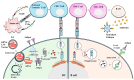
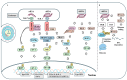
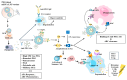
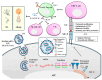
Messenger RNA (mRNA)-based therapeutics have shown remarkable progress in the treatment and prevention of diseases. Lipid nanoparticles (LNPs) have shown great successes in delivering mRNAs. After an mRNA-LNP vaccine enters a cell via an endosome, mRNA is translated into an antigen, which can activate adaptive immunity. mRNAs can bind to various pattern recognition receptors (PRRs), including toll-like receptors (TLRs), and increase the production of inflammatory cytokines. This review summarizes mechanisms of innate immunity induced by mRNAs. Polyethylene glycol (PEG) has been employed as a component of the mRNA-LNP vaccine. PEGylated nanoparticles display enhanced stability by preventing aggregation of particles. However, PEGylation can cause adverse reactions, including blood clearance (ABC) of nanoparticles via complement activation and anaphylaxis. Mechanisms of PEG-induced ABC phenomenon and anaphylaxis are presented and discussed. There have been studies aimed at reducing immune responses associated with PEG to make safe and effective vaccines. Effects of modifying or replacing PEG in reducing immune responses associated with PEGylated nanoparticles are also discussed. Modifying mRNA can induce immune tolerance, which can prevent hypersensitivity reactions induced by PEGylated mRNA-LNP vaccines. Current progress of immune tolerance induction in association with mRNA-LNP is also summarized. This review might be helpful for developing safe and effective PEGylated mRNA-LNP vaccines.
Keywords: ABC phenomenon; PEG; allergies; complement; immune tolerance; innate immunity; mRNA-LNPs.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Berraondo P., Cuesta R., Sanmamed M.F., Melero I. Immunogenicity and Efficacy of Personalized Adjuvant mRNA Cancer Vaccines. Cancer Discov. 2024;14:2021–2024. doi: 10.1158/2159-8290.CD-24-1196. - DOI - PubMed
-
- Maher S., Assaly N.M.E., Aly D.M., Atta S., Fteah A.M., Badawi H., Zahran M.Y., Kamel M. Comparative study of neutralizing antibodies titers in response to different types of COVID-19 vaccines among a group of egyptian healthcare workers. Virol. J. 2024;21:277. doi: 10.1186/s12985-024-02546-0. - DOI - PMC - PubMed
-
- Kumar S., Hsiao Y.W., Wong V.H.Y., Aubin D., Wang J.H., Lisowski L., Rakoczy E.P., Li F., Alarcon-Martinez L., Gonzalez-Cordero A., et al. Characterization of RNA editing and gene therapy with a compact CRISPR-Cas13 in the retina. Proc. Natl. Acad. Sci. USA. 2024;121:e2408345121. doi: 10.1073/pnas.2408345121. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

