Immunobridging Trials: An Important Tool to Protect Vulnerable and Immunocompromised Patients Against Evolving Pathogens
- PMID: 39852798
- PMCID: PMC11768488
- DOI: 10.3390/vaccines13010019
Immunobridging Trials: An Important Tool to Protect Vulnerable and Immunocompromised Patients Against Evolving Pathogens
Abstract
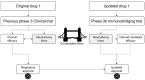
Safeguarding patients from emerging infectious diseases demands strategies that prioritise patient well-being and protection. Immunobridging is an established trial methodology which has been increasingly employed to ensure patient protection and provide clinicians with swift access to vaccines. It uses immunological markers to infer the effectiveness of a new drug through a surrogate measure of efficacy. Recently, this method has also been employed to authorise novel drugs, such as COVID-19 vaccines, and this article explores the concepts behind immunobridging trials, their advantages, issues, and significance in the context of COVID-19 and other infectious diseases. Our goal is to improve awareness among clinicians, patient groups, regulators, and health leaders of the opportunities and issues of immunobridging, so that fewer patients are left without protection from infectious diseases, particularly from major pathogens that may emerge.
Keywords: clinical trials; immunobridging; immunocompromise; monoclonal antibodies; vaccines.
Conflict of interest statement
PC has received honorarium from AstraZeneca. SB has received fees for advisory work from AstraZeneca, GSK, Bayer. TB has received honorarium from MSD, AstraZeneca, Gilead, GSK, Medison. GH is a recipient of research grants from Allovir, Karius, Regeneron, the Cystic Fibrosis Foundation, and AstraZeneca. GH also serves on the scientific advisory boards of Karius, AstraZeneca, and SNIPR BIOME and has received honoraria from MDOutlook, PeerView Institute for Medical Education, and PRIME Inc. YM has received honorarium from Pfizer, AstraZeneca, MSD, Medison, GSK, and Gilead. AP has received advisory board fees from AstraZeneca. FR has received honorarium from AstraZeneca, GSK and Roche. DS has received honoraria from Sanofi, AstraZeneca, and Caredx. LL has received honorarium from Pfizer, AstraZeneca.
Figures
References
-
- Starkey T., Ionescu M.C., Tilby M., Little M., Burke E., Fittall M.W., Khan S., Liu J.K.H., Platt J.R., Mew R., et al. A population-scale temporal case–control evaluation of COVID-19 disease phenotype and related outcome rates in patients with cancer in England (UKCCP) Sci. Rep. 2023;13:11327. doi: 10.1038/s41598-023-36990-9. - DOI - PMC - PubMed
-
- Donken R., Dobson S.R.M., Marty K.D., Cook D., Sauvageau C., Gilca V., Dionne M., McNeil S., Krajden M., Money D., et al. Immunogenicity of 2 and 3 Doses of the Quadrivalent Human Papillomavirus Vaccine up to 120 Months Postvaccination: Follow-up of a Randomized Clinical Trial. Clin. Infect. Dis. 2020;71:1022–1029. doi: 10.1093/cid/ciz887. - DOI - PMC - PubMed
-
- Essink B., Sabharwal C., Cannon K., Frenck R., Lal H., Xu X., Sundaraiyer V., Peng Y., Moyer L., Pride M.W., et al. Pivotal Phase 3 Randomized Clinical Trial of the Safety, Tolerability, and Immunogenicity of 20-Valent Pneumococcal Conjugate Vaccine in Adults Aged ≥18 Years. Clin. Infect. Dis. 2022;75:390–398. doi: 10.1093/cid/ciab990. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources