Antigen Delivery Platforms for Next-Generation Coronavirus Vaccines
- PMID: 39852809
- PMCID: PMC11769099
- DOI: 10.3390/vaccines13010030
Antigen Delivery Platforms for Next-Generation Coronavirus Vaccines
Abstract
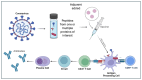
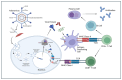
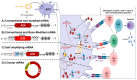
The COVID-19 pandemic, caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), is in its sixth year and is being maintained by the inability of current spike-alone-based COVID-19 vaccines to prevent transmission leading to the continuous emergence of variants and sub-variants of concern (VOCs). This underscores the critical need for next-generation broad-spectrum pan-Coronavirus vaccines (pan-CoV vaccine) to break this cycle and end the pandemic. The development of a pan-CoV vaccine offering protection against a wide array of VOCs requires two key elements: (1) identifying protective antigens that are highly conserved between passed, current, and future VOCs; and (2) developing a safe and efficient antigen delivery system for induction of broad-based and long-lasting B- and T-cell immunity. This review will (1) present the current state of antigen delivery platforms involving a multifaceted approach, including bioinformatics, molecular and structural biology, immunology, and advanced computational methods; (2) discuss the challenges facing the development of safe and effective antigen delivery platforms; and (3) highlight the potential of nucleoside-modified mRNA encapsulated in lipid nanoparticles (LNP) as the platform that is well suited to the needs of a next-generation pan-CoV vaccine, such as the ability to induce broad-based immunity and amenable to large-scale manufacturing to safely provide durable protective immunity against current and future Coronavirus threats.
Keywords: LNP; SARS-CoV-2; antigen delivery platform; antigen delivery system; mRNA; pan-Coronavirus vaccine; srRNA.
Conflict of interest statement
LBM has an equity interest in TechImmune, LLC., a company that may potentially benefit from the research results and serves on the company’s Scientific Advisory Board. LBM’s relationship with TechImmune, LLC., has been reviewed and approved by the University of California, Irvine by its conflict-of-interest policies.
Figures
References
-
- Prakash S., Srivastava R., Coulon P.G., Dhanushkodi N.R., Chentoufi A.A., Tifrea D.F., Edwards R.A., Figueroa C.J., Schubl S.D., Hsieh L., et al. Genome-Wide B Cell, CD4(+), and CD8(+) T Cell Epitopes That Are Highly Conserved between Human and Animal Coronaviruses, Identified from SARS-CoV-2 as Targets for Preemptive Pan-Coronavirus Vaccines. bioRxiv. 2020 reprint in J. Immunol. 2021, 206, 2566–2582. - PMC - PubMed
-
- Coulon P.-G., Prakash S., Dhanushkodi N.R., Srivastava R., Zayou L., Tifrea D.F., Edwards R.A., Figueroa C.J., Schubl S.D., Hsieh L., et al. High frequencies of alpha common cold coronavirus/SARS-CoV-2 cross-reactive functional CD4+ and CD8+ memory T cells are associated with protection from symptomatic and fatal SARS-CoV-2 infections in unvaccinated COVID-19 patients. Front. Immunol. 2024;15:1343716. doi: 10.3389/fimmu.2024.1343716. - DOI - PMC - PubMed
-
- Nguyen D.C., Hentenaar I.T., Morrison-Porter A., Solano D., Haddad N.S., Castrillon C., Runnstrom M.C., Lamothe P.A., Andrews J., Roberts D., et al. SARS-CoV-2-specific plasma cells are not durably established in the bone marrow long-lived compartment after mRNA vaccination. Nat. Med. 2024 doi: 10.1038/s41591-024-03278-y. - DOI - PMC - PubMed
-
- Ishimaru H., Nishimura M., Shigematsu H., Marini M.I., Hasegawa N., Takamiya R., Iwata S., Mori Y. Epitopes of an antibody that neutralizes a wide range of SARS-CoV-2 variants in a conserved subdomain 1 of the spike protein. J. Virol. 2024;98:e0041624. doi: 10.1128/jvi.00416-24. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

