An mRNA-Based Respiratory Syncytial Virus Vaccine Elicits Strong Neutralizing Antibody Responses and Protects Rodents Without Vaccine-Associated Enhanced Respiratory Disease
- PMID: 39852831
- PMCID: PMC11768429
- DOI: 10.3390/vaccines13010052
An mRNA-Based Respiratory Syncytial Virus Vaccine Elicits Strong Neutralizing Antibody Responses and Protects Rodents Without Vaccine-Associated Enhanced Respiratory Disease
Abstract
Background: Respiratory syncytial virus (RSV) causes the most common type of severe lower respiratory tract infection worldwide, and the fusion (F) protein is a target for neutralizing antibodies and vaccine development. This study aimed to investigate the immunogenicity and efficacy of an mRNA-based RSV vaccine with an F protein sequence.
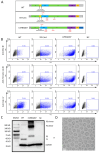
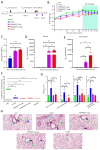
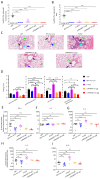
Methods: We designed an mRNA construct encoding a modified RSV F protein, which was further developed into an LNP-encapsulated mRNA vaccine (LVRNA007). LVRNA007 was administered to mice and cotton rats, followed by immunogenicity analysis and viral challenge studies. Protection of rodents from the viral infection was evaluated based on the presence of the virus in the lung and pathological examination of respiratory tissues.
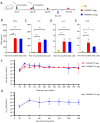
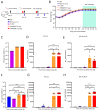
Results: LVRNA007 induced robust humoral and cellular immune responses in both mice and cotton rats, with neutralization antibody levels in the immunized animals maintained at high levels for over one year. Vaccination of LVRNA007 also protected the rodents from RSV challenge, judged by the much decreased virus titer and the pathological score in the lung tissue. In addition, no vaccine-enhanced disease (VED) phenomenon was observed with LVRNA007 vaccination.
Conclusions: Based on the preclinical immunogenicity and efficacy data, LVRNA007 could be a potential promising vaccine for prophylaxis of RSV infection.
Keywords: F protein; LVRNA007; RSV; mRNA vaccine; viral challenge study.
Conflict of interest statement
The authors declare no competing interests. All authors are employees of the company Liverna Therapeutics Inc., Zhuhai, Guangdong, China. Liverna Therapeutics Inc. had a role in the study design, data collection and interpretation, and the decision to submit the work for publication.
Figures
References
-
- Nguyen-Van-Tam J.S., O’Leary M., Martin E.T., Heijnen E., Callendret B., Fleischhackl R., Comeaux C., Tran T.M.P., Weber K. Burden of respiratory syncytial virus infection in older and high-risk adults: A systematic review and meta-analysis of the evidence from developed countries. Eur. Respir. Rev. 2022;31:220105. doi: 10.1183/16000617.0105-2022. - DOI - PMC - PubMed
-
- Li Y., Wang X., Blau D.M., Caballero M.T., Feikin D.R., Gill C.J., Madhi S.A., Omer S.B., Simoes E.A.F., Campbell H., et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet. 2022;399:2047–2064. doi: 10.1016/S0140-6736(22)00478-0. - DOI - PMC - PubMed
-
- Azzari C., Baraldi E., Bonanni P., Bozzola E., Coscia A., Lanari M., Manzoni P., Mazzone T., Sandri F., Checcucci Lisi G., et al. Epidemiology and prevention of respiratory syncytial virus infections in children in Italy. Ital. J. Pediatr. 2021;47:198. doi: 10.1186/s13052-021-01148-8. - DOI - PMC - PubMed
-
- Pellegrinelli L., Galli C., Bubba L., Cereda D., Anselmi G., Binda S., Gramegna M., Pariani E. Respiratory syncytial virus in influenza-like illness cases: Epidemiology and molecular analyses of four consecutive winter seasons (2014–2015/2017–2018) in Lombardy (Northern Italy) J. Med. Virol. 2020;92:2999–3006. doi: 10.1002/jmv.25917. - DOI - PubMed
LinkOut - more resources
Full Text Sources

