T4 Phage Displaying Dual Antigen Clusters Against H3N2 Influenza Virus Infection
- PMID: 39852849
- PMCID: PMC11769387
- DOI: 10.3390/vaccines13010070
T4 Phage Displaying Dual Antigen Clusters Against H3N2 Influenza Virus Infection
Abstract
Background: The current H3N2 influenza subunit vaccine exhibits weak immunogenicity, which limits its effectiveness in preventing and controlling influenza virus infections.
Methods: In this study, we aimed to develop a T4 phage-based nanovaccine designed to enhance the immunogenicity of two antigens by displaying the HA1 and M2e antigens of the H3N2 influenza virus on each phage nanoparticle. Specifically, we fused the Soc protein with the HA1 antigen and the Hoc protein with the M2e antigen, assembling them onto a T4 phage that lacks Soc and Hoc proteins (Soc-Hoc-T4), thereby constructing a nanovaccine that concurrently presents both HA1 and M2e antigens.
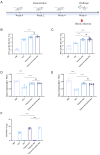
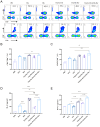
Results: The analysis of the optical density of the target protein bands indicated that each particle could display approximately 179 HA1 and 68 M2e antigen molecules. Additionally, animal experiments demonstrated that this nanoparticle vaccine displaying dual antigen clusters induced a stronger specific immune response, higher antibody titers, a more balanced Th1/Th2 immune response, and enhanced CD4+ and CD8+ T cell effects compared to immunization with HA1 and M2e antigen molecules alone. Importantly, mice immunized with the T4 phage displaying dual antigen clusters achieved full protection (100% protection) against the H3N2 influenza virus, highlighting its robust protective efficacy.
Conclusions: In summary, our findings indicate that particles based on a T4 phage displaying antigen clusters exhibit ideal immunogenicity and protective effects, providing a promising strategy for the development of subunit vaccines against various viruses beyond influenza.
Keywords: HA1 and M2e antigen molecule clusters; T4 phage nanovaccine; immune response.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Ferdinands J.M., Gaglani M., Martin E.T., Middleton D., Monto A.S., Murthy K., Silveira F.P., Talbot H.K., Zimmerman R., Alyanak E., et al. Prevention of Influenza Hospitalization Among Adults in the United States, 2015-2016: Results From the US Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN) J. Infect. Dis. 2019;220:1265–1275. doi: 10.1093/infdis/jiy723. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

