Species Differences in the Biotransformation of Aflatoxin B1: Primary Determinants of Relative Carcinogenic Potency in Different Animal Species
- PMID: 39852983
- PMCID: PMC11768628
- DOI: 10.3390/toxins17010030
Species Differences in the Biotransformation of Aflatoxin B1: Primary Determinants of Relative Carcinogenic Potency in Different Animal Species
Abstract
It has been known since the early days of the discovery of aflatoxin B1 (AFB1) that there were large species differences in susceptibility to AFB1. It was also evident early on that AFB1 itself was not toxic but required bioactivation to a reactive form. Over the past 60 years there have been thousands of studies to delineate the role of ~10 specific biotransformation pathways of AFB1, both phase I (oxidation, reduction) and phase II (hydrolysis, conjugation, secondary oxidations, and reductions of phase I metabolites). This review provides a historical context and substantive analysis of each of these pathways as contributors to species differences in AFB1 hepatoxicity and carcinogenicity. Since the discovery of AFB1 as the toxic contaminant in groundnut meal that led to Turkey X diseases in 1960, there have been over 15,000 publications related to aflatoxins, of which nearly 8000 have addressed the significance of biotransformation (metabolism, in the older literature) of AFB1. While it is impossible to give justice to all of these studies, this review provides a historical perspective on the major discoveries related to species differences in the biotransformation of AFB1 and sets the stage for discussion of other papers in this Special Issue of the important role that AFB1 metabolites have played as biomarkers of exposure and effect in thousands of human studies on the toxic effects of aflatoxins. Dr. John Groopman has played a leading role in every step of the way-from initial laboratory studies on specific AFB1 metabolites to the application of molecular biomarkers in epidemiological studies associating dietary AFB1 exposure with liver cancer, and the design and conduct of chemoprevention clinical trials to reduce cancer risk from unavoidable aflatoxin exposures by alteration of specific AFB1 biotransformation pathways. This article is written in honor of Dr. Groopman's many contributions in this area.
Keywords: aflatoxin; biomarkers; biotransformation; cytochrome P450; glutathione S-transferase; species differences.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




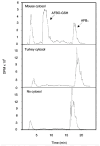

References
-
- Ayres J.L., Lee D.J., Wales J.H., Sinnhuber R.O. Aflatoxin structure and hepatocarcinogenicity in rainbow trout (Salmo gairdneri) J. Natl. Cancer Inst. 1971;46:561–564. - PubMed
-
- Lee D.J., Wales J.H., Sinnhuber R.O. Promotion of aflatoxin-induced hepatoma growth in trout by methyl malvalate and sterculate. Cancer Res. 1971;31:960–963. - PubMed
-
- Wogan G.N., Newberne P.M. Dose-response characteristics of aflatoxin B1 carcinogenesis in the rat. Cancer Res. 1967;27:2370–2376. - PubMed
-
- Roebuck B.D., Wogan G.N. Species comparison of in vitro metabolism of aflatoxin B1. Cancer Res. 1977;37:1649–1656. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

