Nivolumab and rituximab in treatment-naïve follicular lymphoma: the phase 2 1st FLOR study
- PMID: 39853272
- PMCID: PMC11960644
- DOI: 10.1182/bloodadvances.2024015487
Nivolumab and rituximab in treatment-naïve follicular lymphoma: the phase 2 1st FLOR study
Abstract
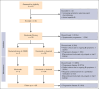
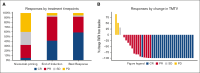
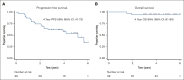
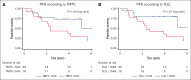
Follicular lymphoma (FL) outcomes are influenced by host immune activity. CD20-directed therapy plus programmed cell death 1 inhibition (PD-1i) increases T-cell tumor killing and natural killer cell antibody-dependent cell cytotoxicity. Mounting evidence supports immune priming using PD-1i before cancer directed agents. Our multicenter, phase 2 1st FLOR study enrolled 39 patients with previously untreated advanced-stage FL to receive 4 cycles of nivolumab (240 mg), then 4 cycles of 2-weekly nivolumab plus rituximab 375 mg/m2 (induction), then 1 year of monthly nivolumab (480 mg) plus 2 years of 2-monthly rituximab maintenance. Participants with complete response (CR) after nivolumab priming continued nivolumab monotherapy. The primary end point was toxicity during induction. Adverse events of grade ≥3 during induction occurred in 33% (n = 13); most commonly elevated amylase/lipase (15%), liver enzyme derangement (11%), and infection (10%). Three patients discontinued nivolumab secondary to toxicity. Overall response rate was 92% (CR, 59%). Median follow-up was 51 months. Median and 4-year progression-free survival (PFS) were 61 months (95% confidence interval [CI], 2-72) and 58% (95% CI, 34-97); 70% of responders remained in CR. The 4-year overall survival was 95%. High baseline total metabolic tumor volume (TMTV) and total lesion glycolysis conferred inferior PFS (P = .04 and P = .02). Additionally, high baseline tumor CD8A gene expression was associated with improved PFS (P = .03). Nivolumab priming followed by nivolumab-rituximab in treatment-naïve FL is associated with favorable toxicity and high response rates, potentially providing an alternative to chemotherapy. TMTV and high tumor CD8A expression are promising immunotherapy biomarkers for FL. This trial was registered at www.ClinicalTrials.gov as #NCT03245021.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.B. reports honoraria from advisory board participation with Roche, Novartis, BeiGene, and Gilead. G.C. reports research funding from Bristol Myers Squibb, HUTCHMED, Regeneron, Amgen, Roche, Merck, AstraZeneca, Pharmacyclics, Bayer, Incyte, and Dizal Pharma, and consultant/advisory roles with Bristol Myers Squibb, Regeneron, and Takeda. D.L. reports honoraria/advisory roles with Roche and Gilead. K.M. reports honoraria from/advisory board participation with BeiGene and AbbVie. A.M.S. reports research funding (paid to institution) from EMD Serono, ITM, Telix Pharmaceuticals, Avid Radiopharmaceuticals, Fusion Pharmaceuticals, Cyclotek, MedImmune, Antengene, Humanigen, and Telix Pharmaceuticals, and advisory board roles with Imagion and ImmunOs. C.K. reports honoraria from/consultancy with Roche, Takeda, Gilead, AstraZeneca, Merck Sharpe & Dohme, and BeiGene. E.A.H. reports research funding (paid to institution) from Roche, Bristol Myers Squibb, Merck KGaA, AstraZeneca, TG Therapeutics, and Merck; consultant or advisory roles with Roche (paid to institution), Merck Sharpe & Dohme (paid to institution), AstraZeneca (paid to institution), Gilead, Antengene (paid to institution), Novartis (paid to institution), Regeneron, Janssen (paid to institution), Specialised Therapeutics (paid to institution), and Sobi; and reports travel expenses from AstraZeneca. The remaining authors declare no competing financial interests.
Figures


References
-
- The Non-Hodgkin's Lymphoma Classification Project A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. Blood. 1997;89(11):3909–3918. - PubMed
-
- Junlén HR, Peterson S, Kimby E, et al. Follicular lymphoma in Sweden: nationwide improved survival in the rituximab era, particularly in elderly women: a Swedish Lymphoma Registry study. Leukemia. 2015;29(3):668–676. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

