Engineered Extracellular Vesicles Modified by Angiopep-2 Peptide Promote Targeted Repair of Spinal Cord Injury and Brain Inflammation
- PMID: 39853366
- PMCID: PMC11803916
- DOI: 10.1021/acsnano.4c14675
Engineered Extracellular Vesicles Modified by Angiopep-2 Peptide Promote Targeted Repair of Spinal Cord Injury and Brain Inflammation
Abstract
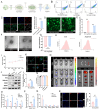
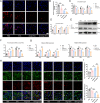
Engineered extracellular vesicles play an increasingly important role in the treatment of spinal cord injury. In order to prepare more effective engineered extracellular vesicles, we biologically modified M2 microglia. Angiopep-2 (Ang2) is an oligopeptide that can target the blood-brain barrier. Through single-cell sequencing and immunofluorescence experiments, we confirmed that the expression of LRP-1, the targeted receptor of Ang2, was elevated after spinal cord injury. Subsequently, we integrated the Ang2 peptide segment into M2 microglia to obtain Ang2-EVs, which could successfully target the site of spinal cord injury. However, in order to improve the function of Ang2-EVs, we pretreated M2 microglia with melatonin, which has anti-inflammatory effects, to obtain M-Ang2-EVs. The results of single-nucleus sequencing of the mouse spinal cord verified that neurons and OPCs gradually transformed into subtypes related to nerve repair functions after treatment with M-Ang2-EVs. This is consistent with the sequencing and enrichment analysis of miRNAs contained in M-Ang2-EVs. We further verified through experiments that M-Ang2-EVs can promote microglia/macrophages to phagocytose sphingomyelin, promote axon remyelination and axon elongation, and maintain the integrity of the blood-spinal barrier. Since Ang2 can also target the blood-brain barrier, we found that M-Ang2-EVs can also reduce brain inflammation that results from spinal cord injury. Our study applied the Angiopep-2 peptide to spinal cord injury to enhance the targeting of injured cells, and successfully construct engineered extracellular vesicles that can target the spinal cord injury site and the brain.
Keywords: angiopep-2; axonal regeneration; cerebral inflammation; engineered extracellular vesicles; melatonin; spinal cord injury.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Anjum A.; Yazid M. D.; Fauzi Daud M.; Idris J.; Ng A. M. H.; Selvi Naicker A.; Ismail O. H. R.; Athi Kumar R. K.; Lokanathan Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21 (20), 7533.10.3390/ijms21207533. - DOI - PMC - PubMed
-
- Nakazaki M.; Morita T.; Lankford K. L.; Askenase P. W.; Kocsis J. D. Small extracellular vesicles released by infused mesenchymal stromal cells target M2 macrophages and promote TGF-β upregulation, microvascular stabilization and functional recovery in a rodent model of severe spinal cord injury. J. Extracell. Vesicles 2021, 10 (11), e1213710.1002/jev2.12137. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

