Treatment of peri-implant mucositis using an Er:YAG laser or an ultrasonic device: a randomized, controlled clinical trial
- PMID: 39853624
- PMCID: PMC11759739
- DOI: 10.1186/s40729-025-00591-0
Treatment of peri-implant mucositis using an Er:YAG laser or an ultrasonic device: a randomized, controlled clinical trial
Erratum in
-
Correction: Treatment of peri-implant mucositis using an Er:YAG laser or an ultrasonic device: a randomized, controlled clinical trial.Int J Implant Dent. 2025 Feb 26;11(1):15. doi: 10.1186/s40729-025-00599-6. Int J Implant Dent. 2025. PMID: 40009124 Free PMC article. No abstract available.
Abstract
Purpose: The study assessed the clinical outcomes following treatment of peri-implant mucositis using Er:YAG laser or an ultrasonic device over six months. Patients' experience of pain, aesthetics, and Quality of life were further assessed.
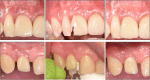
Methods: One dental implant, per included patient, diagnosed with peri-implant mucositis underwent treatment with an Er:YAG laser (test) or an ultrasonic scaler (control) randomly. Treatments were performed at baseline and months three and six. At each session, oral hygiene was instructed after plaque registration, and the patient was guided in proper cleaning technique using a toothbrush and interproximal aids as needed. Full mouth bleeding on probing (FMBoP), full mouth plaque score (FMPS), implant bleeding on probing (BoP), implant mean graded bleeding (mBI), implant probing pocket depts (PPD), implant suppuration and bone levels were assessed. Oral health-related Quality of life (OHQoL) and visual analog scales (VAS), which reflect aesthetic satisfaction and pain of the treatment, were also evaluated.
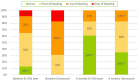
Results: Forty-six patients were included. FMBoP was significantly reduced from 30.1 to 21.5% (test) (p < 0.001) respectively from 35.0% to 30% (control) (p < 0.01). FMPS showed significant reduction from 61.5 to 32.7% (test) (p < 0.001) and from 58.7 to 39.1% (control) (p < 0.001). At the implant BoP reduced from 89.0 to 55.7% (test) (p < 0.001) respectively from 94.9 to 63.7% (control) (p < 0.001). mBI was reduced from 1.3 to 0.6 (test) (p < 0.01) and from 1.9 to 0.8 (control) (p < 0.001). Distribution of "no bleeding" increased from 13 to 61% (test) (p < 0.05) and from 0 to 35% (control) (p < 0.05). At month three, statistically significant intergroup differences were shown for PPD ≥ 4 mm with 43.5% (test) respectively 73.9% (control) (p < 0.05). At month six, statistically significant intergroup differences, were shown for FMBoP 21.5% (test) respectively 30% (control) (p < 0.05) and for plaque score at the implant 4.0% (test) respectively 26% (control) (p < 0.05). Less pain was reported in the laser group at three days 0.08 (test) respectively 0.2 (control) (p < 0.05).
Conclusions: Treatment of peri-implant mucositis was effective regardless of whether the treatment was performed with an Er:YAG laser or an ultrasonic scaler. Fewer diseased sites were diagnosed at six months following laser treatment.
Trial registration: Registered at www.
Clinicaltrials: gov : study no, NCT05772299.
Keywords: Er:YAG laser; Patient-reported outcome measures; Peri-implant mucositis; Professional mechanical plaque removal; Ultrasonic scaler.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This longitudinal clinical study was approved by the Regional Ethics Board in Uppsala (Dnr 2020-02586). All the subjects signed an informed consent form to have their data used for scientific purposes. The study was conducted in accordance with the Declaration of Helsinki in 1975 revised in 2013. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Efficacy of mechanical/physical approaches for implant surface decontamination in non-surgical submarginal instrumentation of peri-implantitis. A systematic review.J Clin Periodontol. 2023 Jun;50 Suppl 26:188-211. doi: 10.1111/jcpe.13762. Epub 2023 Jan 23. J Clin Periodontol. 2023. PMID: 36550060
-
Non-surgical therapy of peri-implant mucositis-Mechanical/physical approaches: A systematic review.J Clin Periodontol. 2023 Jun;50 Suppl 26:135-145. doi: 10.1111/jcpe.13789. Epub 2023 Feb 28. J Clin Periodontol. 2023. PMID: 36802083
-
Comparison of the efficacy of Er,Cr:YSGG laser on oral biofilm removal from implant surfaces with various application times for the treatment of peri-implantitis defects: ex vivo study.BMC Oral Health. 2024 Aug 22;24(1):980. doi: 10.1186/s12903-024-04698-5. BMC Oral Health. 2024. PMID: 39174958 Free PMC article. Clinical Trial.
-
Accuracy of Clinical Parameters in Predicting/Diagnosing Peri-Implant Bone Loss.J Clin Periodontol. 2025 Aug;52(8):1070-1081. doi: 10.1111/jcpe.14095. Epub 2025 May 4. J Clin Periodontol. 2025. PMID: 40320760 Free PMC article.
-
Soft-Tissue Phenotype Modification as an Adjunct to the Treatment of Peri-Implant Mucositis-A Quasi-Randomized Clinical Trial.J Periodontal Res. 2025 Jul 28. doi: 10.1111/jre.70014. Online ahead of print. J Periodontal Res. 2025. PMID: 40717582
References
-
- Heitz-Mayfield LJA, Needleman I, Salvi GEB. Consensus statements and clinical recommendations for preventing and managing biologic and technical implant complications. Int J Oral Maxillofac Implants. 2014;29:346–50. - PubMed
-
- Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42(Suppl 16):158–71. - PubMed
-
- Heitz-Mayfield LJA, Salvi GE. Peri-implant mucositis. J Clin Periodontol. 2018;45(Suppl 20):237–45. - PubMed
-
- Renvert S, Polyzois I. Treatment of pathologic peri-implant pockets. Periodontol. 2000;2018(76):180–90. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

