Capecitabine and temozolomide or temozolomide alone in patients with atypical carcinoids
- PMID: 39853630
- PMCID: PMC12069480
- DOI: 10.1007/s12020-025-04171-5
Capecitabine and temozolomide or temozolomide alone in patients with atypical carcinoids
Abstract
Background: Lung neuroendocrine neoplasms (NENs) represent about 20% of all lung cancers. Few therapeutic options are available for atypical carcinoids (ACs). Single-agent temozolomide (TEM) is active in lung NENs, but whether the addition of capecitabine (CAPTEM) is associated with improved outcomes, is unknown. We sought to investigate the TEM-based therapies (TEM or CAPTEM) in patients with advanced AC.
Material and methods: This was a retrospective analysis of prospectively collected data from patients with AC of the lung referred to our institution from January 2003 to January 2023 who have received chemotherapy with either TEM or CAPTEM as any line treatment. Primary endpoint was progression free survival (PFS), secondary endpoints included overall response rate (ORR) and overall survival (OS).
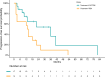
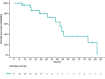
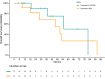
Results: In this study we included 31 patients with advanced AC. Median Ki-67 was 14.4% (3-30). CAPTEM in 17 patients (54.8%), while TEM was administered in 14 patients (45.2%). Overall, ORR was 39% (N = 12/31, all partial responses), while median PFS and OS were 57.4 months (95%CI: 43.2-71.7) and 24.4 months (95% confidence interval [95%CI]: 14.7-34.1). Median PFS was 33.9 months (15.6-52.1) in the CAPTEM group, while it was 15.5 (7.3-23.8) in the TEM group (p = 0.047). When adjusting for potential confounding factors, treatment with TEM vs CAPTEM retained its independent association with an increased risk of progression (HR: 4.01 [95%CI: 1.18-13.68]; p = 0.027).
Conclusions: Treatment with CAPTEM is associated with longer PFS than TEM alone in patients with AC. Prospective studies with larger sample size are needed to validate this finding.
Keywords: Atypical carcinoids; CAPTEM; Chemotherapy; Lung neuroendocrine neoplasms; TEM.
© 2025. The Author(s).
Conflict of interest statement
Compliance with ethical standards. Conflict of interest: F.G. received honoraria for speaker/advisory roles from Servier, Eli Lilly, Iqvia, Merck Serono, Amgen, and Bristol-Myers Squibb outside the present work. D.C. received honoraria for speaker/advisory roles from Ipsen, Novartis and Esteve outside the present work. F.P., A.L.S., G.L., L.G., A.Z., S.P., and S.O.: no competing interests. Ethics approval: The study was approved by local IRB (Comitato Etico indipendente, IRCCS Policlinico Sant’Orsola-Malpighi of Bologna, protocol code: SOCRATE, 787/2019/Oss/AOUBo) and was conducted in accordance with the principles of the Declaration of Helsinki (revision of Edinburgh, 2000).
Figures

References
-
- P.L. Filosso, O. Rena, F. Guerrera, P. Moreno Casado, D. Sagan, F. Raveglia et al. Clinical management of atypical carcinoid and large-cell neuroendocrine carcinoma: a multicentre study on behalf of the European association of thoracic surgeons (ESTS) neuroendocrine tumours of the lung working group. Eur. J. Cardiothorac. Surg. 48(luglio 1), 55–64 (2015) - DOI - PubMed
-
- M.E. Caplin, E. Baudin, P. Ferolla, P. Filosso, M. Garcia-Yuste, E. Lim et al. Pulmonary neuroendocrine (carcinoid) tumors: European neuroendocrine tumor society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann. Oncol. 26(agosto 8), 1604–1620 (2015) - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

