Nutritional Interventions to Attenuate Quadriceps Muscle Deficits following Anterior Cruciate Ligament Injury and Reconstruction
- PMID: 39853659
- PMCID: PMC11985700
- DOI: 10.1007/s40279-025-02174-w
Nutritional Interventions to Attenuate Quadriceps Muscle Deficits following Anterior Cruciate Ligament Injury and Reconstruction
Abstract
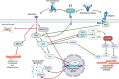
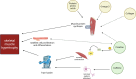
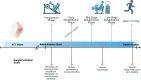
Following anterior cruciate ligament (ACL) injury, quadriceps muscle atrophy persists despite rehabilitation, leading to loss of lower limb strength, osteoarthritis, poor knee joint health and reduced quality of life. However, the molecular mechanisms responsible for these deficits in hypertrophic adaptations within the quadriceps muscle following ACL injury and reconstruction are poorly understood. While resistance exercise training stimulates skeletal muscle hypertrophy, attenuation of these hypertrophic pathways can hinder rehabilitation following ACL injury and reconstruction, and ultimately lead to skeletal muscle atrophy that persists beyond ACL reconstruction, similar to disuse atrophy. Numerous studies have documented beneficial roles of nutritional support, including nutritional supplementation, in maintaining and/or increasing muscle mass. There are three main mechanisms by which nutritional supplementation may attenuate muscle atrophy and promote hypertrophy: (1) by directly affecting muscle protein synthetic machinery; (2) indirectly increasing an individual's ability to work harder; and/or (3) directly affecting satellite cell proliferation and differentiation. We propose that nutritional support may enhance rehabilitative responses to exercise training and positively impact molecular machinery underlying muscle hypertrophy. As one of the fastest growing knee injuries worldwide, a better understanding of the potential mechanisms involved in quadriceps muscle deficits following ACL injury and reconstruction, and potential benefits of nutritional support, are required to help restore quadriceps muscle mass and/or strength. This review discusses our current understanding of the molecular mechanisms involved in muscle hypertrophy and disuse atrophy, and how nutritional supplements may leverage these pathways to maximise recovery from ACL injury and reconstruction.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: N.J.H., A.J.S.J., L.M.B., and D.A.O. declare that this manuscript was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. M.J.S. is the owner of a company, Straight Up Performance, that produces supplements, including collagen peptides. Author contributions: M.J.S., N.J.H., A.J.S.J., L.M.B. and D.A.O. conceived the manuscript. M.J.S. conducted the literature search and wrote the first draft of the manuscript. M.J.S., N.J.H., A.J.S.J., L.M.B. and D.A.O. critically revised and contributed to the manuscript. M.J.S., N.J.H., A.J.S.J., L.M.B. and D.A.O. read and approved the final manuscript. Funding: The authors’ research is supported by an Australian Government Research Training Program (RTP) Scholarship and a Collaborative Research Grant and PhD Top-up Grant from the Defence Science Institute. No external funding was received for the preparation and writing of this manuscript. Availability statement: All data generated or analysed during this study are included in this published article.
Figures
References
-
- Mather 3rd RC, Koenig L, Kocher MS, Dall TM, Gallo P, Scott DJ, et al. Societal and economic impact of anterior cruciate ligament tears. J Bone Jt Surg Am [Internet]. 2013;95(19):1751–9. https://pubmed.ncbi.nlm.nih.gov/24088967. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

