A randomized sequential cross-over trial evaluating five purportedly ICP-lowering drugs in idiopathic intracranial hypertension
- PMID: 39853738
- PMCID: PMC11794974
- DOI: 10.1111/head.14897
A randomized sequential cross-over trial evaluating five purportedly ICP-lowering drugs in idiopathic intracranial hypertension
Abstract
Objective: To gain initial insight into the efficacy to lower intracranial pressure (ICP), side effects, and effects on cognition of five drugs commonly used to treat idiopathic intracranial hypertension (IIH).
Background: Limited clinical data exist for the treatment for IIH. Impaired cognition is recognized in IIH and can be exacerbated by medications.
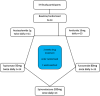
Methods: This human experimental medicine study was a secondary analysis that focused on an unblinded randomized, sequential, cross-over extension of a previously completed randomized controlled trial. This study evaluated females with active IIH, recruited from University Hospital Birmingham, UK. Participants were treated, in randomized order, for 2 weeks with acetazolamide, amiloride, furosemide, spironolactone, and topiramate; assessment was at baseline and 2 weeks with a minimum 1-week drug washout between drugs. The primary outcome was change in ICP at 2 weeks post-drug administration. The cognitive evaluation was an exploratory study of the trial. ICP was recorded with telemetric, intraparenchymal ICP monitors (Raumedic, Hembrechts, Germany). Adverse events were recorded, and cognition was assessed utilizing the National Institutes of Health Toolbox Cognitive Battery.
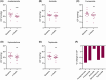
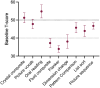
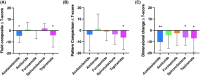
Results: Fourteen participants were recruited and evaluated by intention-to-treat analysis. Mean (standard deviation) body mass index was 37.3 (7.0) kg/m2 and ICP was 33.2 (7.1) cm cerebrospinal fluid (CSF) at baseline. ICP fell with four drugs (mean [standard error (SE)]), acetazolamide -3.3 (1.0) mmHg, p = 0.001, furosemide -3.0 (0.9) mmHg, p = 0.001, spironolactone -2.7 (0.9) mmHg, p = 0.003, and topiramate -2.3 (0.9) mmHg, p = 0.010. There was no significant difference between drugs. Side effects were common with acetazolamide (100%, 11/11) and topiramate (93%, 13/14). Baseline cognitive performance was impaired, T-score (mean [SE]) 37.2 (2.6). After treatment, there was a further significant reduction in the fluid cognition domain (ability to process and integrate) with acetazolamide (mean T-score [SE]), -5.0 (2.6), p = 0.057 and topiramate -4.1 (2.0), p = 0.061.
Conclusions: Acetazolamide, furosemide, spironolactone, and topiramate marginally reduced ICP. While their effects were not significant, this study was not powered to detect a difference between drugs. Participants reported significant side effects with acetazolamide and topiramate including cognitive decline. Cognitive measures were impaired by acetazolamide and topiramate. Therapeutics with greater efficacy and a favorable side effect profile are an unmet need in the treatment of IIH.
Keywords: adverse events; cerebrospinal fluid; cognition; idiopathic intracranial hypertension; intracranial pressure; therapeutics.
© 2025 The Author(s). Headache: The Journal of Head and Face Pain published by Wiley Periodicals LLC on behalf of American Headache Society.
Conflict of interest statement
Figures

References
-
- Ball AK, Howman A, Wheatley K, et al. A randomised controlled trial of treatment for idiopathic intracranial hypertension. J Neurol. 2010;258(5):874‐881. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

