Congenital Titinopathy: Comprehensive Characterization of the Most Severe End of the Disease Spectrum
- PMID: 39853809
- PMCID: PMC11889535
- DOI: 10.1002/ana.27087
Congenital Titinopathy: Comprehensive Characterization of the Most Severe End of the Disease Spectrum
Abstract
Congenital titinopathy has recently emerged as one of the most common congenital muscle disorders.
Objective: To better understand the presentation and clinical needs of the under-characterized extreme end of the congenital titinopathy severity spectrum.
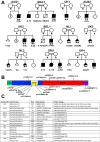
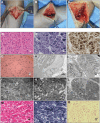
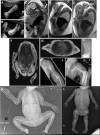
Methods: We comprehensively analyzed the clinical, imaging, pathology, autopsy, and genetic findings in 15 severely affected individuals from 11 families.
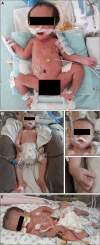
Results: Prenatal features included hypokinesia or akinesia and growth restriction. Six pregnancies were terminated. Nine infants were born at or near term with severe-to-profound weakness and required resuscitation. Seven died following withdrawal of life support. Two surviving children require ongoing respiratory support. Most cohort members had at least 1 disease-causing variant predicted to result in some near-normal-length titin expression. The exceptions, from 2 unrelated families, had homozygous truncating variants predicted to induce complete nonsense mediated decay. However, subsequent analyses suggested that the causative variant in each family had an additional previously unrecognized impact on splicing likely to result in some near-normal-length titin expression. This impact was confirmed by minigene assay for 1 variant.
Interpretation: This study confirms the clinical variability of congenital titinopathy. Severely affected individuals succumb prenatally/during infancy, whereas others survive into adulthood. It is likely that this variability is because of differences in the amount and/or length of expressed titin. If confirmed, analysis of titin expression could facilitate clinical prediction and increasing expression might be an effective treatment strategy. Our findings also further-support the hypothesis that some near-normal-length titin expression is essential to early prenatal survival. Sometimes expression of normal/near-normal-length titin is due to disease-causing variants having an additional impact on splicing. ANN NEUROL 2025;97:611-628.
© 2025 The Author(s). Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Nothing to report.
Figures

References
-
- Whiting A, Wardale J, Trinick J. Does titin regulate the length of muscle thick filaments? J Mol Biol 1989;205:263–268. - PubMed
MeSH terms
Substances
Grants and funding
- MRC and BRC Neuromuscular Centre Biobank
- GNT1090428/Australian NHMRC Neil Hamilton Fairley Early Career Research Fellowship
- Muscular Dystrophy UK
- National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust
- Belgian Kids' Fund for Pediatric Research
- Belgian Royal Academy of Medicine
- GNT2002640/Australian NHMRC Ideas Grant
- Belgian Association Against Neuromuscular Diseases
- GNT1122952/Australian NHMRC Fellowship
- Luminesce Alliance - Innovation for Children's Health
- NHS England Highly Specialised Services to the Dubowitz Neuromuscular Centre
- Fonds Erasme
- New South Wales State Government Research Attraction and Acceleration Program
- University College London
- GNT1176265/Australian NHMRC Investigator Grant
LinkOut - more resources
Full Text Sources

