Effects of Exercise Training on the Bone Marrow Immune Microenvironment and Minimal Residual Disease in Multiple Myeloma Patients Following First-Line Treatment
- PMID: 39853819
- PMCID: PMC11760657
- DOI: 10.1111/sms.70020
Effects of Exercise Training on the Bone Marrow Immune Microenvironment and Minimal Residual Disease in Multiple Myeloma Patients Following First-Line Treatment
Abstract
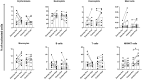
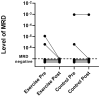
The purpose of the study was to investigate the effects of exercise training on the bone marrow immune microenvironment and on minimal residual disease of multiple myeloma patients who completed first-line induction treatment. Eight multiple myeloma patients underwent 5 months of exercise training along with standard medical treatment. Eight age- and sex-matched patients who received medical treatment only, served as controls. Before and after the intervention, white blood cells, red blood cells, and platelets, as well as the percentages of neutrophils, lymphocytes, monocytes, eosinophils, and basophils, were measured in the peripheral blood. Abnormal plasma cells, normal plasma cells, B cells, T cells, NK/NKT cells, monocytes, neutrophils, eosinophils, basophils, mast cells, myeloid progenitors, erythroid progenitors, and erythroblasts were assessed in the bone marrow. Exercise training increased the percentage of blood monocytes (mean difference 3.5% ± 2.6%; p = 0.006), while no change was detected in the control group. In the bone marrow, the CD27+ T cell subset increased (mean difference 18.2% ± 21.9%; p = 0.043) and the ratio of CD27-/CD27+ T lymphocytes decreased (pre: 1.06 ± 0.59; post: 0.76 ± 0.47; p = 0.049) in the exercise group, but remained unaltered in the control group. In conclusion, the study provides evidence that 5 months of exercise training can induce an increase in the percentage of activated T lymphocytes, as shown by the higher expression of the costimulatory CD27 marker. It also suggests that exercise-induced changes in the bone marrow microenvironment may be beneficial in the control of clonal cell proliferation.
Keywords: CD27 receptor; bone marrow immunity; exercise immunology; exercise oncology immunology; exercise training; minimal residual disease; multiple myeloma; sports medicine.
© 2025 The Author(s). Scandinavian Journal of Medicine & Science In Sports published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Methodological considerations for the high sensitivity detection of multiple myeloma measurable residual disease.Cytometry B Clin Cytom. 2020 Mar;98(2):161-173. doi: 10.1002/cyto.b.21862. Epub 2019 Dec 23. Cytometry B Clin Cytom. 2020. PMID: 31868315 Free PMC article.
-
Adoptive transfer of activated marrow-infiltrating lymphocytes induces measurable antitumor immunity in the bone marrow in multiple myeloma.Sci Transl Med. 2015 May 20;7(288):288ra78. doi: 10.1126/scitranslmed.aaa7014. Sci Transl Med. 2015. PMID: 25995224 Free PMC article. Clinical Trial.
-
Early alterations in stem-like/resident T cells, innate and myeloid cells in the bone marrow in preneoplastic gammopathy.JCI Insight. 2019 Apr 23;5(11):e127807. doi: 10.1172/jci.insight.127807. JCI Insight. 2019. PMID: 31013254 Free PMC article.
-
Role of Immunotherapy in Targeting the Bone Marrow Microenvironment in Multiple Myeloma: An Evolving Therapeutic Strategy.Pharmacotherapy. 2017 Jan;37(1):129-143. doi: 10.1002/phar.1871. Epub 2017 Jan 6. Pharmacotherapy. 2017. PMID: 27870103 Review.
-
The Role of Marrow Microenvironment in the Growth and Development of Malignant Plasma Cells in Multiple Myeloma.Int J Mol Sci. 2021 Apr 24;22(9):4462. doi: 10.3390/ijms22094462. Int J Mol Sci. 2021. PMID: 33923357 Free PMC article. Review.
References
-
- Kumar S., Paiva B., Anderson K. C., et al., “International Myeloma Working Group Consensus Criteria for Response and Minimal Residual Disease Assessment in Multiple Myeloma,” Lancet Oncology 17 (2016): e328–e346. - PubMed
-
- Rajkumar S. V., Dimopoulos M. A., Palumbo A., et al., “International Myeloma Working Group Updated Criteria for the Diagnosis of Multiple Myeloma,” Lancet Oncology 15 (2014): e538–e548. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

