Natural Killer Cell Education in Women With Recurrent Pregnancy Loss
- PMID: 39853841
- PMCID: PMC11760664
- DOI: 10.1111/aji.70045
Natural Killer Cell Education in Women With Recurrent Pregnancy Loss
Abstract
Problem: Natural killer (NK) cells undergo education for full functionality via interactions between killer immunoglobulin-like receptors (KIRs) or NKG2A and human leukocyte antigen (HLA) ligands. Presumably, education is important during early pregnancy as insufficient education has been associated with impaired vascular remodeling and restricted fetal growth in mice. NK cell education is influenced by receptor co-expression patterns, human cytomegalovirus (CMV), the HLA-ER107G dimorphism, and HLA-B leader peptide variants. We hypothesized altered NK cell education status and differences in frequencies of HLA-E genotypes and HLA-B leader peptide variants in women with recurrent pregnancy loss (RPL) compared to women with previously uncomplicated pregnancies, and between CMV seropositive and seronegative RPL women.
Methods of study: Peripheral blood mononuclear cells were analyzed by flow cytometry. HLA-ABC was typed by sequence-specific oligonucleotide PCR, and HLA-E by Sanger sequencing. CMV status was determined by anti-CMV IgG immunoassay. NK cells were considered "educated" if the HLA ligand to a KIR or NKG2A was present.
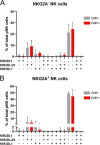
Results: KIR/NKG2A co-expression patterns and percentages of educated NK cells were similar between RPL and controls, and between seropositive and seronegative RPL women. Frequencies of HLA-E genotypes and HLA-B leader peptide variants were comparable. RPL women with the HLA-B T/T variant had a lower percentage of NKG2A-educated NK cells (47.8%) compared to controls (66.4%) (p = 0.025).
Conclusions: HLA-B leader peptide variants might impact NKG2A-specific NK cell education in RPL, warranting validation in larger studies. Follow-up studies are needed to investigate the education status of uterine NK cells and their role in pregnancy.
Keywords: NKG2A; cytomegalovirus; education; killer‐cell immunoglobulin‐like receptor; natural killer cells; recurrent pregnancy loss.
© 2025 The Author(s). American Journal of Reproductive Immunology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Wu M. F. and Raulet D. H., “Class I‐Deficient Hemopoietic Cells and Nonhemopoietic Cells Dominantly Induce Unresponsiveness of Natural Killer Cells to Class I‐Deficient Bone Marrow Cell Grafts,” Journal of Immunology 158, no. 4 (1997): 1628–1633. - PubMed
-
- Chan H.‐W., Miller J. S., Moore M. B., and Lutz C. T., “Epigenetic Control of Highly Homologous Killer Ig‐Like Receptor Gene Alleles,” Journal of Immunology 175, no. 9 (2005): 5966–5974. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

