Artificial Cornea Substitute Based on Hydrogel Skeletons with Natural Stromal Hierarchical Structure and Extracellular Matrix for Sutureless Transplantation
- PMID: 39853921
- PMCID: PMC12097023
- DOI: 10.1002/advs.202411540
Artificial Cornea Substitute Based on Hydrogel Skeletons with Natural Stromal Hierarchical Structure and Extracellular Matrix for Sutureless Transplantation
Abstract
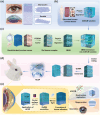
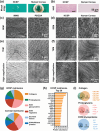
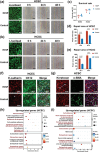
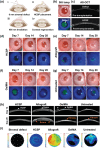
Corneal substitutes with structural and compositional characteristics resembling those of natural corneas have attracted considerable attention. However, biomimicking the complex hierarchical organization of corneal stroma is challenging. In this study, humanized corneal stroma-like adhesive patches (HCSPs) are prepared through a multi-step process. First, polyethylene glycol diacrylate is cast and cured within decellularized porcine cornea (DPC) templates. The DPCs are then enzymatically digested to obtain hydrogel skeletons, which are finally integrated with human corneal extracellular matrix and methacrylate gelatin. HCSPs replicate the ultrastructure, protein components, and optical properties of human corneas and exhibit improved anti-swelling and anti-degradation capabilities compared with conventional DPCs and recombinant human collagen patches. HCSPs can deliver methacrylate gelatin at the ocular surface temperature (37 °C) and achieve stable adhesion to the corneal stroma upon 405 nm light irradiation. Furthermore, HCSPs promote the survival and migration of corneal epithelial and stromal cells while preserving their phenotypes. In rabbit models of lamellar keratoplasty and microperforation repair, HCSPs accelerate epithelial healing, minimize suture-associated complications, and maintain structural stability. These findings suggest that HCSPs are promising donor corneal substitutes for clinical applications.
Keywords: artificial cornea substitutes; biostructure replication; corneal extracellular matrix; hydrogel skeletons; sutureless transplantations.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Natural Extracellular Matrix Scaffold-Based Hydrogel Corneal Patch with Temperature and Light-Responsiveness for Penetrating Keratoplasty and Sutureless Stromal Defect Repair.Adv Healthc Mater. 2025 Apr;14(10):e2402567. doi: 10.1002/adhm.202402567. Epub 2024 Nov 19. Adv Healthc Mater. 2025. PMID: 39558795
-
Corneal stromal structure replicating humanized hydrogel patch for sutureless repair of deep anterior-corneal defect.Biomaterials. 2025 Feb;313:122754. doi: 10.1016/j.biomaterials.2024.122754. Epub 2024 Aug 14. Biomaterials. 2025. PMID: 39197237
-
Decellularized porcine cornea-derived hydrogels for the regeneration of epithelium and stroma in focal corneal defects.Ocul Surf. 2020 Oct;18(4):748-760. doi: 10.1016/j.jtos.2020.07.020. Epub 2020 Aug 22. Ocul Surf. 2020. PMID: 32841745
-
[Transplantation of corneal endothelial cells].Nippon Ganka Gakkai Zasshi. 2002 Dec;106(12):805-35; discussion 836. Nippon Ganka Gakkai Zasshi. 2002. PMID: 12610838 Review. Japanese.
-
Integration and remodelling of a collagen anterior lamellar keratoplasty graft in an animal model - A preliminary report.Exp Eye Res. 2021 Aug;209:108661. doi: 10.1016/j.exer.2021.108661. Epub 2021 Jun 5. Exp Eye Res. 2021. PMID: 34102207 Review.
References
-
- Nosrati H., Ashrafi‐Dehkordi K., Alizadeh Z., Sanami S., Banitalebi‐Dehkordi M., Polim. Med. 2020, 50, 57. - PubMed
-
- Gain P., Jullienne R., He Z., Aldossary M., Acquart S., Cognasse F., Thuret G., JAMA Ophthalmol. 2016, 134, 167. - PubMed
-
- a) Xu Y., Liu J., Song W., Wang Q., Sun X., Zhao Q., Huang Y., Li H., Peng Y., Yuan J., Ji B., Ren L., Adv. Sci. 2023, 10, 2205878; - PMC - PubMed
- b) Kong B., Chen Y., Liu R., Liu X., Liu C., Shao Z., Xiong L., Liu X., Sun W., Mi S., Nat. Commun. 2020, 11, 1435; - PMC - PubMed
- c) Majumdar S., Wang X., Sommerfeld S. D., Chae J. J., Athanasopoulou E. N., Shores L. S., Duan X., Amzel L. M., Stellacci F., Schein O., Guo Q., Singh A., Elisseeff J. H., Adv. Funct. Mater. 2018, 28, 1804076; - PMC - PubMed
- d) Lei M., Zhang S., Zhou H., Wan H., Lu Y., Lin S., Sun J., Qu X., Liu C., ACS Nano 2022, 16, 10632; - PubMed
- e) Kong B., Sun L., Liu R., Chen Y., Shang Y., Tan H., Zhao Y., Sun L., Chem. Eng. J. 2022, 428, 131012.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
