Persistent Effects of Landscape Context on Recruitment Dynamics During Secondary Succession of Tropical Forests
- PMID: 39853923
- PMCID: PMC11758763
- DOI: 10.1111/gcb.70037
Persistent Effects of Landscape Context on Recruitment Dynamics During Secondary Succession of Tropical Forests
Abstract
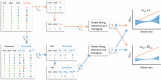
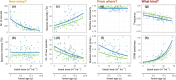
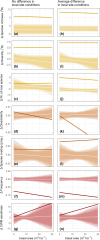
Large-scale reforestation is promoted as an important strategy to mitigate climate change and biodiversity loss. A persistent challenge for efforts to restore ecosystems at scale is how to accelerate ecological processes, particularly natural regeneration. Yet, despite being recognized as an important barrier to the recovery of diverse plant communities in tropical agricultural landscapes, the impacts of dispersal limitation on natural regeneration in secondary forests-and especially how this changes as these forests grow older-are still poorly studied. In a region where animals have been shown to be the dominant seed dispersers, we evaluate the impacts of proximity to a connected network of narrow streamside strips of forest (SSF) on recruitment in 1-40-year-old secondary forests. We used 8 years of annual census data from 45 sites with paired plots, one directly adjoining an SSF and the other further uphill (henceforth "landscape context"), and a null model approach to test the effects of proximity to SSFs and basal area, while accounting for variation in soil, topography, and distance between plots and stand structure. In general, we found that landscape context affects multiple aspects of recruitment, including species diversity and the proportion of rarer and less-widely distributed species among the recruits. Unexpectedly, this effect did not weaken over time, despite a fast increase in stand basal area and diversity. This suggests that forest development over the first decades of succession may not be sufficient to attract the animals that disperse rarer tree species. Our results provide empirical evidence to guide restoration initiatives in agricultural landscapes in tropical regions, principally prioritizing the restoration of forest corridor networks along streams, while also highlighting the knowledge gap about restoring animal dispersers in secondary forests.
Keywords: dispersal corridors; ecological succession; forest fragments; forest restoration; natural regeneration; recruitment dynamics; secondary forest; seed limitation.
Global Change Biology© 2025 The Author(s). Global Change Biology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures

References
-
- Acevedo‐Charry, O. , and Aide T. M.. 2019. “Recovery of Amphibian, Reptile, Bird and Mammal Diversity During Secondary Forest Succession in the Tropics.” Oikos 128, no. 8: 1065–1078. 10.1111/oik.06252. - DOI
-
- Aide, T. M. , and Cavelier J.. 1994. “Barriers to Lowland Tropical Forest Restoration in the Sierra Nevada de Santa Marta, Colombia.” Restoration Ecology 2, no. 4: 219–229. 10.1111/j.1526-100X.1994.tb00054.x. - DOI
-
- Almond, G. , Bignoli D. J., and Petersen T., eds. 2022. Living Planet Report 2022 – Building a Nature‐ Positive Society. Gland, Switzerland: WWF. https://livingplanet.panda.org/en‐GB/.
-
- Anzures‐Dadda, A. , and Manson R. H.. 2007. “Patch‐ and Landscape‐Scale Effects on Howler Monkey Distribution and Abundance in Rainforest Fragments.” Animal Conservation 10, no. 1: 69–76. 10.1111/j.1469-1795.2006.00074.x. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

