Neonatal Nutrition and Brain Structure at 7 Years in Children Born Very Preterm
- PMID: 39853980
- PMCID: PMC11762234
- DOI: 10.1001/jamanetworkopen.2024.56080
Neonatal Nutrition and Brain Structure at 7 Years in Children Born Very Preterm
Abstract
Importance: Neonatal protein intake following very preterm birth has long lasting effects on brain development. However, it is uncertain whether these effects are associated with improved or impaired brain maturation.
Objective: To assess the association of neonatal protein intake following very preterm birth with brain structure at 7 years of age.
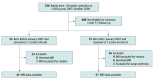
Design, setting, and participants: This cohort study involved children born very preterm before or after a change in neonatal intensive care unit nutritional protocol that increased protein intake at the National Women's Hospital in Auckland, New Zealand. The children completed magnetic resonance imaging (MRI) scanning at 7 years. There were 128 children who were initially eligible. MRI data were ineligible for analysis if excessive head motion or clinical brain abnormalities were present. Data were collected from July 2012 to January 2016, and data analysis took place from January 2017 to March 2024.
Exposure: Neonatal intensive care unit nutritional protocol. Those who were born before the protocol change took place (July 2005 to December 2006) were in the old protocol group, while those who were born after the protocol change (January 2007 to October 2008) were in the new protocol group. Observers were blind to participant grouping.
Main outcomes and measures: All actual enteral and parenteral intakes of protein, fat, energy, and breast milk for days 1 to 7 and days 1 to 14, and growth velocity to postnatal day 28 were calculated for each infant. Preplanned outcomes were group comparisons between regional brain volumes and diffusion parameters of major white matter tracts along with analyses with both groups combined exploring associations of nutrition with brain metrics.
Results: Data from 99 children were analyzed, including 42 in the old protocol group (26 female [55%]; mean [SD] gestational age at birth, 27 [2] weeks) and 57 in the new protocol group (27 female [47%]; mean [SD] gestational age at birth, 26 [2] weeks). Protein intake differed between the groups at both 7 days (old protocol: mean [SD] intake, 17 [2] g/kg-1; new protocol: mean [SD] intake, 21 [2] g/kg-1) and 14 days after birth (old protocol: mean [SD] intake, 41 [6] g/kg-1; new protocol: mean [SD] intake, 45 [7] g/kg-1). The new protocol group had smaller brain volume as a percentage of intercranial volume than the old protocol group (mean [SD], 80% [4%] vs 86% [7%]) but absolute brain volumes were similar. The new protocol group had significantly thinner lateral occipital and lateral parietal cortices than the old protocol group. With both groups combined, those with greater protein, fat, energy, and breast milk intake had more mature diffusion tensor metrics (higher fractional anisotropy and less diffusion) across multiple tracts, although this finding did not reach statistical significance for every tract.
Conclusions and relevance: In this cohort of children born very preterm, children with greater neonatal protein intake had a more mature profile of brain metrics assessed with MRI at 7 years of age. These results contribute to the ongoing evaluation of optimal nutrition for infants born very preterm and suggest that the protein intake experienced by the new protocol group may promote brain maturation in a way that is still observable at 7 years of age.
Conflict of interest statement
Figures
References
-
- Agostoni C, Buonocore G, Carnielli VP, et al. ; ESPGHAN Committee on Nutrition . Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2010;50(1):85-91. doi: 10.1097/MPG.0b013e3181adaee0 - DOI - PubMed