Activation of S1PR2 on macrophages and the hepatocyte S1PR2/RhoA/ROCK1/MLC2 pathway in vanishing bile duct syndrome
- PMID: 39854311
- PMCID: PMC11760576
- DOI: 10.1371/journal.pone.0317568
Activation of S1PR2 on macrophages and the hepatocyte S1PR2/RhoA/ROCK1/MLC2 pathway in vanishing bile duct syndrome
Abstract
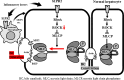
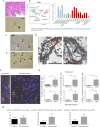
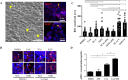
Immunologic bile duct destruction is a pathogenic condition associated with vanishing bile duct syndrome (VBDS) after liver transplantation and hematopoietic stem-cell transplantation. As the bile acid receptor sphingosine 1-phosphate receptor 2 (S1PR2) plays a critical role in recruitment of bone marrow-derived monocytes/macrophages to sites of cholestatic liver injury, S1PR2 expression was examined using cultured macrophages and patient tissues. Bile canaliculi destruction precedes intrahepatic ductopenia; therefore, we focused on hepatocyte S1PR2 and the downstream RhoA/Rho kinase 1 (ROCK1) signaling pathway and bile canaliculi alterations using three-dimensional hepatocyte culture models that form obvious bile canaliculus-like networks. Multiplex immunohistochemistry revealed increased numbers of S1PR2+CD45+CD68+FCN1+ inflammatory macrophages and S1PR2+CD45+CD68+MARCO+ Kupffer cells in liver tissues showing ductopenia due to graft-versus-host disease and rejection post-liver transplant compared with normal liver. Macrophage expression of proinflammatory cytokines, including MCP1, was reduced following S1PR2 inhibition. Taurocholic acid and S1P2 agonist induced hepatocyte S1PR2 and reduced RhoA/ROCK1 expression, resulting in bile canaliculi dilatation. S1PR2 inhibition reversed the effect on RhoA/ROCK1 expression, resulting in maintenance of bile canaliculi through myosin light chain 2 (MLC2) phosphorylation. Activation of S1PR2 on macrophages and S1PR2 on hepatocytes may disrupt bile canaliculi dynamics in VBDS under regulation by RhoA/ROCK1 through MLC2 phosphorylation.
Copyright: © 2025 Miyagawa-Hayashino et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
SS, MH, and HO are employees of SCREEN Holdings Co., Ltd. This does not alter our adherence to PLOS ONE policies on sharing data and materials. The other authors have declared that no competing interests exist.
Figures


Similar articles
-
S1PR2/RhoA/ROCK1 pathway promotes inflammatory bowel disease by inducing intestinal vascular endothelial barrier damage and M1 macrophage polarization.Biochem Pharmacol. 2022 Jul;201:115077. doi: 10.1016/j.bcp.2022.115077. Epub 2022 May 10. Biochem Pharmacol. 2022. PMID: 35537530
-
Rho-kinase/myosin light chain kinase pathway plays a key role in the impairment of bile canaliculi dynamics induced by cholestatic drugs.Sci Rep. 2016 May 12;6:24709. doi: 10.1038/srep24709. Sci Rep. 2016. PMID: 27169750 Free PMC article.
-
S1PR2 Mediates Smooth Muscle Cell Proliferation and Endothelial Cell Permeability via Akt/mTOR and RhoA/ROCK1 in Atherosclerosis.J Biochem Mol Toxicol. 2025 Jun;39(6):e70351. doi: 10.1002/jbt.70351. J Biochem Mol Toxicol. 2025. PMID: 40495705
-
Vanishing bile duct syndrome in Hodgkin's lymphoma: A case report and literature review.World J Gastroenterol. 2017 Jan 14;23(2):366-372. doi: 10.3748/wjg.v23.i2.366. World J Gastroenterol. 2017. PMID: 28127210 Free PMC article. Review.
-
Bile Acid-Activated Receptors: GPBAR1 (TGR5) and Other G Protein-Coupled Receptors.Handb Exp Pharmacol. 2019;256:19-49. doi: 10.1007/164_2019_230. Handb Exp Pharmacol. 2019. PMID: 31302759 Review.
Cited by
-
Biological Actions of Bile Acids via Cell Surface Receptors.Int J Mol Sci. 2025 May 22;26(11):5004. doi: 10.3390/ijms26115004. Int J Mol Sci. 2025. PMID: 40507815 Free PMC article. Review.
-
Low density of intratumoral M1-macrophage infiltration may correlate with worse prognosis in low-grade early-stage uterine endometrioid carcinoma.Gynecol Oncol Rep. 2025 May 10;59:101758. doi: 10.1016/j.gore.2025.101758. eCollection 2025 Jun. Gynecol Oncol Rep. 2025. PMID: 40487855 Free PMC article.
References
-
- Basturk O, Volkan Adsay N. Bile duct diseases. In: Burt AD, Ferrell LD, Hubscher SG, editors. MacSween’s Pathology of the Liver. Amsterdam, Netherlands: Elsevier, Ltd.; 2024. pp.556–643.
-
- Clouston A, Hubscher S. Transplantation Pathology. In Burt AD, Ferrell LD, Hubscher SG, editors. MacSween’s Pathology of the Liver. Amsterdam, Netherlands: Elsevier, Ltd.; 2024. pp.947–1038.
-
- Ishii I, Ye X, Friedman B, Kawamura S, Contos JJ, Kingsbury MA, et al.. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P(2)/LP(B2)/EDG-5 and S1P(3)/LP(B3)/EDG-3. J Biol Chem. 2002; 277: 25152–25159. doi: 10.1074/jbc.M200137200 - DOI - PubMed
-
- Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol. 2003;23: 1534–1545. doi: 10.1128/MCB.23.5.1534-1545.2003 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

