ZDHHC2 promoted antimycobacterial responses by selective autophagic degradation of B-RAF and C-RAF in macrophages
- PMID: 39854453
- PMCID: PMC11758995
- DOI: 10.1126/sciadv.adq7706
ZDHHC2 promoted antimycobacterial responses by selective autophagic degradation of B-RAF and C-RAF in macrophages
Abstract
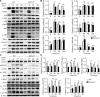
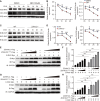
S-Palmitoylation is a reversible post-translational modification involving saturated fatty acid palmitate-to-cysteine linkage in the protein, which guides many aspects of macrophage physiology in health and disease. However, the precise role and underlying mechanisms of palmitoylation in Mycobacterium tuberculosis infection of macrophages remain elusive. Here, we found that M. tuberculosis infection induced the expression of zinc-finger DHHC domain-type palmitoyl-transferases (ZDHHCs), particularly ZDHHC2, in mouse macrophages. Furthermore, ZDHHC2 deficiency in mouse macrophages impaired the immunity against M. tuberculosis and reduced the production of various proinflammatory cytokines. Mechanistic studies revealed the involvement of ZDHHC2 in mediating the palmitoylation of B-RAF and C-RAF, affecting their autophagic degradation and stabilizing protein levels. The increased abundance of B-RAF and C-RAF subsequently increases the activity of the extracellular signal-regulated kinase (ERK) signaling pathway, affecting the survival of M. tuberculosis within macrophages. These findings suggest that ZDHHC2 is a potential target for treating tuberculosis.
Figures





References
-
- Bagcchi S., WHO's Global Tuberculosis Report 2022. Lancet. Microbe 4, E20 (2023). - PubMed
-
- Du X., Sheng J., Chen Y., He S., Yang Y., Huang Y., Fu Y., Lie L., Han Z., Zhu B., Liu H., Wen Q., Zhou X., Zhou C., Hu S., Ma L., The E3 ligase HERC5 promotes antimycobacterial responses in macrophages by ISGylating the phosphatase PTEN. Sci. Signal. 16, eabm1756 (2023). - PubMed
-
- Jankute M., Cox J. A., Harrison J., Besra G. S., Assembly of the mycobacterial cell wall. Annu. Rev. Microbiol. 69, 405–423 (2015). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

