Cryo-EM structure and regulation of human NAD kinase
- PMID: 39854463
- PMCID: PMC11759006
- DOI: 10.1126/sciadv.ads2664
Cryo-EM structure and regulation of human NAD kinase
Abstract
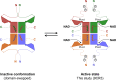
Reduced nicotinamide adenine dinucleotide phosphate (NADPH) is a crucial reducing cofactor for reductive biosynthesis and protection from oxidative stress. To fulfill their heightened anabolic and reductive power demands, cancer cells must boost their NADPH production. Progrowth and mitogenic protein kinases promote the activity of cytosolic NAD kinase (NADK), which produces NADP+, a limiting NADPH precursor. However, the molecular architecture and mechanistic regulation of human NADK remain undescribed. Here, we report the cryo-electron microscopy structure of human NADK, both in its apo-form and in complex with its substrate NAD+ (nicotinamide adenine dinucleotide), revealing a tetrameric organization with distinct structural features. We discover that the amino (N)- and carboxyl (C)-terminal tails of NADK have opposing effects on its enzymatic activity and cellular NADP(H) levels. Specifically, the C-terminal region is critical for NADK activity, whereas the N-terminal region exhibits an inhibitory role. This study highlights molecular insights into the regulation of a vital enzyme governing NADP(H) production.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

