Mature chromatin packing domains persist after RAD21 depletion in 3D
- PMID: 39854464
- PMCID: PMC11759041
- DOI: 10.1126/sciadv.adp0855
Mature chromatin packing domains persist after RAD21 depletion in 3D
Abstract
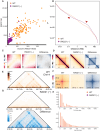
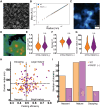
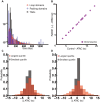
Understanding chromatin organization requires integrating measurements of genome connectivity and physical structure. It is well established that cohesin is essential for TAD and loop connectivity features in Hi-C, but the corresponding change in physical structure has not been studied using electron microscopy. Pairing chromatin scanning transmission electron tomography with multiomic analysis and single-molecule localization microscopy, we study the role of cohesin in regulating the conformationally defined chromatin nanoscopic packing domains. Our results indicate that packing domains are not physical manifestation of TADs. Using electron microscopy, we found that only 20% of packing domains are lost upon RAD21 depletion. The effect of RAD21 depletion is restricted to small, poorly packed (nascent) packing domains. In addition, we present evidence that cohesin-mediated loop extrusion generates nascent domains that undergo maturation through nucleosome posttranslational modifications. Our results demonstrate that a 3D genomic structure, composed of packing domains, is generated through cohesin activity and nucleosome modifications.
Figures






References
-
- Soochit W., Sleutels F., Stik G., Bartkuhn M., Basu S., Hernandez S. C., Merzouk S., Vidal E., Boers R., Boers J., van der Reijden M., Geverts B., van Cappellen W. A., van den Hout M., Ozgur Z., van IJcken W. F. J., Gribnau J., Renkawitz R., Graf T., Houtsmuller A., Grosveld F., Stadhouders R., Galjart N., CTCF chromatin residence time controls three-dimensional genome organization, gene expression and DNA methylation in pluripotent cells. Nat. Cell Biol. 23, 881–893 (2021). - PubMed
-
- Arnould C., Rocher V., Saur F., Bader A. S., Muzzopappa F., Collins S., Lesage E., Le Bozec B., Puget N., Clouaire T., Mangeat T., Mourad R., Ahituv N., Noordermeer D., Erdel F., Bushell M., Marnef A., Legube G., Chromatin compartmentalization regulates the response to DNA damage. Nature 623, 183–192 (2023). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

