Sulfur dioxide exposure of mice induces peribronchiolar fibrosis-A defining feature of deployment-related constrictive bronchiolitis
- PMID: 39854594
- PMCID: PMC11761160
- DOI: 10.1371/journal.pone.0313992
Sulfur dioxide exposure of mice induces peribronchiolar fibrosis-A defining feature of deployment-related constrictive bronchiolitis
Abstract
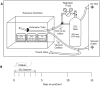
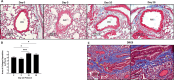
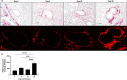
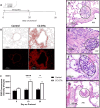
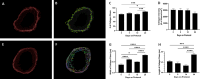
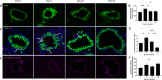
Deployment-related constrictive bronchiolitis (DRCB) has emerged as a health concern in military personnel returning from Southwest Asia. Exposure to smoke from a fire at the Al-Mishraq sulfur enrichment facility and/or burn pits was reported by a subset of Veterans diagnosed with this disorder. DRCB is characterized by thickening and fibrosis of small airways (SA) in the lung, but whether these are related to toxin inhalation remains uncertain. The aim of this study was to determine whether sulfur dioxide (SO2) exposure can induce histopathological features of DRCB. C57BL/6J mice were exposed to 50 ± 5 ppm SO2 for one hour/day for five consecutive days. Lungs from exposed and unexposed mice were evaluated on day 5, 10, and 20. Lung sections were stained using hematoxylin and eosin, Masson's trichrome, picrosirius red (PSR), and immunofluorescence for club cell secretory protein, acetylated-α-tubulin, and Ki67. Small airway wall thickness was determined by morphometric analysis and collagen content was quantified by measuring PSR fluorescence intensity. CurveAlign and CT-FIRE were used to enumerate collagen fibers and assess fibers' width and length, respectively. Leukocyte subpopulations were quantified by flow cytometry analysis. This protocol of SO2 exposure of mice: 1) Triggered club cell proliferation and differentiation; 2) Increased SA wall thickness by inducing subepithelial collagen deposition; and 3) Increased width, length, and number, but not density, of collagen fibers within the wall of SA. 4) Induced no peribronchiolar inflammation or respiratory bronchiolitis. Collectively, these findings implicate club cell proliferation and differentiation in the profibrotic response to SO2 and identify this SO2 exposure as a potentially effective though imperfect model for studying SA fibrosis in DRCB.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- National Academies of Sciences Engineering and Medicine. 2020. Respiratory health effects of airborne hazards exposures in the southwest Asia theater of military operations. Washington, DC: The National Academies Press. - PubMed
-
- Self-reported illness and health status among Gulf War veterans. A population-based study. The Iowa Persian Gulf Study Group. JAMA. 1997;277(3):238–45. Epub 1997/01/15. . - PubMed
-
- Kelsall HL, Sim MR, Forbes AB, McKenzie DP, Glass DC, Ikin JF, et al. Respiratory health status of Australian veterans of the 1991 Gulf War and the effects of exposure to oil fire smoke and dust storms. Thorax. 2004;59(10):897–903. Epub 2004/09/30. doi: 10.1136/thx.2003.017103 [pii]. ; PubMed Central PMCID: PMC1746848. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

