Prevalence of FGFR2b Protein Overexpression in Advanced Gastric Cancers During Prescreening for the Phase III FORTITUDE-101 Trial
- PMID: 39854659
- PMCID: PMC11781561
- DOI: 10.1200/PO-24-00710
Prevalence of FGFR2b Protein Overexpression in Advanced Gastric Cancers During Prescreening for the Phase III FORTITUDE-101 Trial
Erratum in
-
Erratum: Prevalence of FGFR2b Protein Overexpression in Advanced Gastric Cancers During Prescreening for the Phase III FORTITUDE-101 Trial.JCO Precis Oncol. 2025 Apr;9:e2500223. doi: 10.1200/PO-25-00223. Epub 2025 Apr 3. JCO Precis Oncol. 2025. PMID: 40179328 Free PMC article. No abstract available.
Abstract
Purpose: Fibroblast growth factor receptor 2 isoform IIIb (FGFR2b) protein overexpression is an emerging biomarker in gastric cancer and gastroesophageal junction cancer (GC). We assessed FGFR2b protein overexpression prevalence in nearly 3,800 tumor samples as part of the prescreening process for a global phase III study in patients with newly diagnosed advanced or metastatic GC.
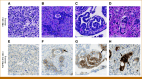
Methods: As of June 28, 2024, 3,782 tumor samples from prescreened patients from 37 countries for the phase III FORTITUDE-101 trial (ClinicalTrials.gov identifier: NCT05052801) were centrally tested for FGFR2b protein overexpression by immunohistochemistry (IHC) and had evaluable results. FGFR2b positivity was defined as both any % tumor cells (TC) and ≥10% TC exhibiting moderate-to-strong (2+/3+) membranous FGFR2b staining. Prevalence was analyzed across patient and sample characteristics.
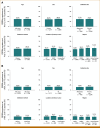
Results: FGFR2b protein overexpression at any % and ≥10%, 2+/3+ TC positivity was 37.8% (1,428/3,782 [95% CI, 36.2 to 39.3]) and 16.2% (612/3,782 [95% CI, 15 to 17.4]), respectively. Of any %, 2+/3+ TC-positive tumors, 42.9% (612/1,428 [95% CI, 40.3 to 45.4]) were FGFR2b ≥10%, 2+/3+ TC positive. FGFR2b prevalence was not notably different within multiple patient and sample characteristics examined (age, sex, collection method [biopsy v resection], collection site, location of primary tumor, and geographic region).
Conclusion: As of the data cutoff date, we report the largest prevalence assessment of FGFR2b protein overexpression in GC with more than one third (37.8%) of patients with GC exhibiting FGFR2b protein overexpression (any % TC, 2+/3+) by a validated IHC assay. Approximately 16% of patients had FGFR2b protein overexpression in ≥10% of TC. FGFR2b prevalence was similar across geographic regions and within defined patient and sample variables regardless of the level of expression.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures


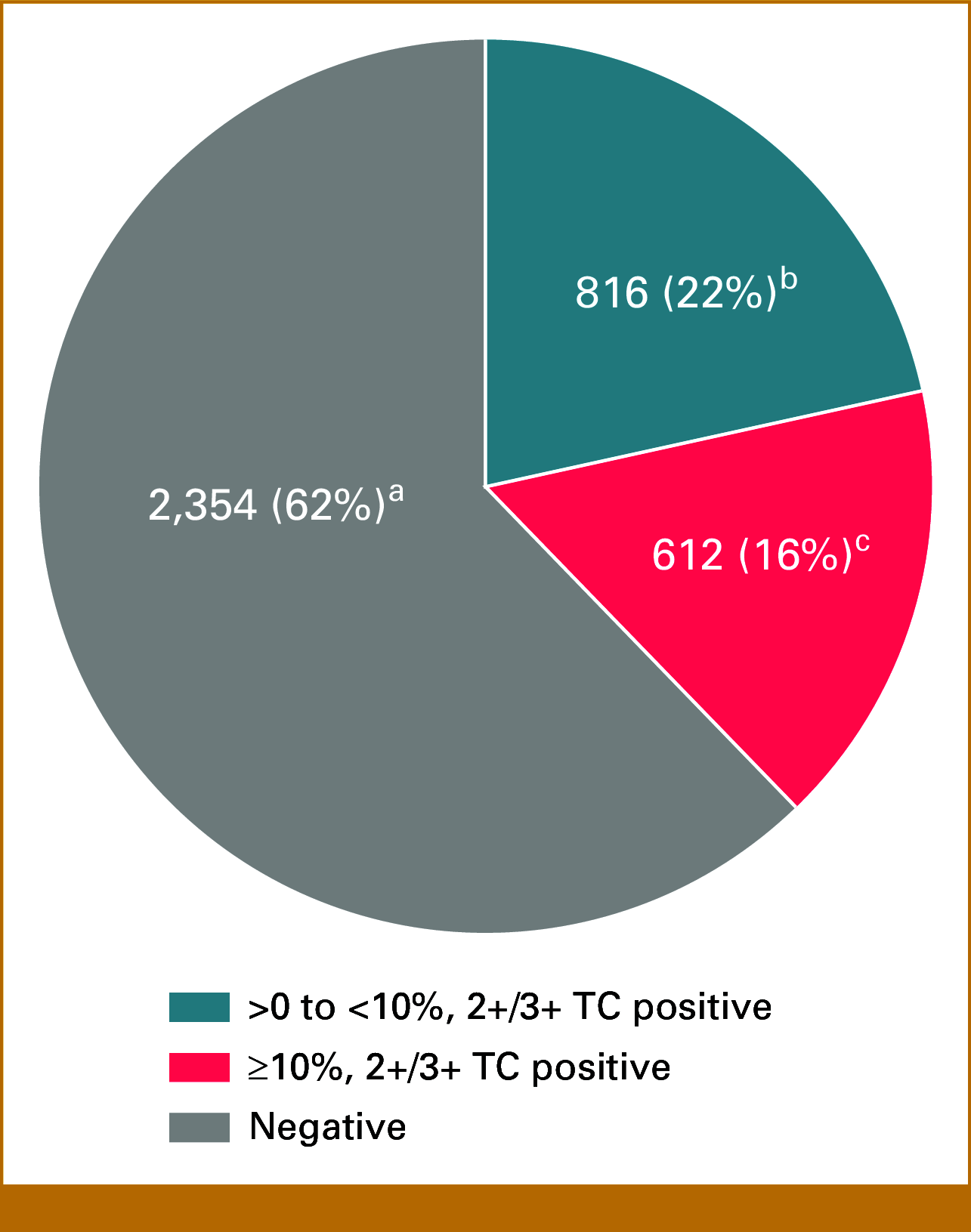
References
-
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. - PubMed
-
- Lordick F, Carneiro F, Cascinu S, et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005–1020. - PubMed
-
- Gullo I, Carneiro F, Oliveira C, et al. Heterogeneity in gastric cancer: From pure morphology to molecular classifications. Pathobiology. 2017;85:50–63. - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

