Pertuzumab Retreatment for Human Epidermal Growth Factor Receptor 2-Positive Locally Advanced/Metastatic Breast Cancer (PRECIOUS Study): Final Overall Survival Analysis
- PMID: 39854662
- PMCID: PMC12058362
- DOI: 10.1200/JCO-24-01673
Pertuzumab Retreatment for Human Epidermal Growth Factor Receptor 2-Positive Locally Advanced/Metastatic Breast Cancer (PRECIOUS Study): Final Overall Survival Analysis
Erratum in
-
Erratum: Pertuzumab Retreatment for Human Epidermal Growth Factor Receptor 2-Positive Locally Advanced/Metastatic Breast Cancer (PRECIOUS Study): Final Overall Survival Analysis.J Clin Oncol. 2025 May 10;43(14):1749. doi: 10.1200/JCO-25-00722. Epub 2025 Apr 11. J Clin Oncol. 2025. PMID: 40215433 Free PMC article. No abstract available.
Abstract
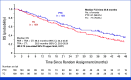
In the primary analysis of the open-label phase III PRECIOUS study, pertuzumab retreatment combined with trastuzumab plus chemotherapy of physician's choice (PTC) significantly improved investigator-assessed progression-free survival (PFS) compared with trastuzumab plus physician's choice chemotherapy (TC) in patients with human epidermal growth factor receptor 2 (HER2)-positive locally advanced/metastatic breast cancer (LA/mBC). Here, we report final overall survival (OS) at the median follow-up of 25.8 months. Patients who have previously received pertuzumab-containing regimens as first-/second-line treatment for LA/mBC were randomly assigned 1:1 to two groups, PTC group (n = 110) and TC group (n = 109). Median OS was longer in the PTC group (median OS 36.2 v 26.5 months; hazard ratio [HR], 0.73 [one side 95% CI upper limit, 0.97]). Updated median investigator-assessed PFS (5.5 v 4.2 months; HR, 0.81 [one side 95% CI upper limit, 1.02]) were also better in the PTC group. Median PFS by independent review did not show the difference between the two groups (4.4 v 4.4 months; HR, 1.03 [one side 95% CI upper limit, 1.36]). These findings suggest that dual HER2 blockade with pertuzumab plus trastuzumab could contribute to improving OS in patients who have previously been treated with pertuzumab-containing regimens for HER2-positive LA/mBC.
Trial registration: ClinicalTrials.gov NCT02514681.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures



References
-
- Giordano SH, Franzoi MAB, Temin S, et al. Systemic therapy for advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO guideline update. J Clin Oncol. 2022;40:2612–2635. - PubMed
-
- Yamamoto Y, Iwata H, Ueno T, et al. A randomized, open-label, phase III trial of pertuzumab retreatment in HER2-positive locally advanced/metastatic breast cancer patients previously treated with pertuzumab, trastuzumab and chemotherapy: The Japan Breast Cancer Research Group-M05 PRECIOUS study. Jpn J Clin Oncol. 2018;48:855–859. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

