Randomised feasibility study of an intestinal adsorbent in acute diarrhoea in The Gambia
- PMID: 39855680
- PMCID: PMC11759216
- DOI: 10.1136/bmjpo-2024-003133
Randomised feasibility study of an intestinal adsorbent in acute diarrhoea in The Gambia
Abstract
Background: Diarrhoea remains a leading cause of death in children. An intestinal adsorbent may reduce diarrhoea duration and severity.
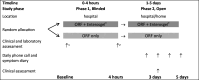
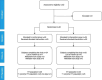
Methods: Randomised controlled feasibility trial with two phases: phase 1 (0-4 hours and double-blind) and phase 2 (up to 5 days and open-label). 50 children aged 6-59 months with acute diarrhoea presenting with no or some dehydration to the emergency paediatric unit and outpatient clinic at Edward Francis Small Teaching Hospital, Banjul, The Gambia were randomised to either standard treatment (oral rehydration fluid and zinc) or standard treatment with polymethylsiloxane polyhydrate for up to 5 days.
Results: Recruitment was completed in 7 months. All but one child completed the study. There were no major protocol deviations although patient-held diaries did not collect reliable information. Time from randomisation to the last watery stool (primary outcome) was shorter in the intervention than control arm (mean difference -19.3 hours, 95% CI -30.9 to -7.8). Stool frequency was lower in the intervention arm on days 2 (95% CI -0.8 to -1.3 to -0.3) and 3 (95% CI -0.8; -1.3 to -0.3). One serious event (death) occurred in the control arm.
Conclusions: A randomised, controlled trial is feasible. Further clinical trials are warranted to confirm the efficacy of polymethylsiloxane polyhydrate in acute diarrhoea and inform management guidelines.
Trial registration number: PACTR202302683128875.
Keywords: Child Health; Gastroenterology; Low and Middle Income Countries.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: CH is Research Director and EM is the Chief Executive Officer for Enteromed, UK the UK supplier of Enterosgel and study sponsor. RH is Chief Medical Officer for Bioline Products s.r.o., the manufacturer of Enterosgel. CH, EM and RH were not involved in undertaking any study procedures in The Gambia including data collection, analysis and interpretation. All other authors declare no potential conflicts of interest.
Figures
Similar articles
-
Gelatine tannate in the management of acute gastroenteritis in children: a randomised controlled trial.BMJ Open. 2018 May 24;8(5):e020205. doi: 10.1136/bmjopen-2017-020205. BMJ Open. 2018. PMID: 29794092 Free PMC article. Clinical Trial.
-
A double-blind placebo-controlled trial of azithromycin to reduce mortality and improve growth in high-risk young children with non-bloody diarrhoea in low resource settings: the Antibiotics for Children with Diarrhoea (ABCD) trial protocol.Trials. 2020 Jan 13;21(1):71. doi: 10.1186/s13063-019-3829-y. Trials. 2020. PMID: 31931848 Free PMC article.
-
Double-blinded randomised placebo controlled trial of enterosgel (polymethylsiloxane polyhydrate) for the treatment of IBS with diarrhoea (IBS-D).Gut. 2022 Dec;71(12):2430-2438. doi: 10.1136/gutjnl-2022-327293. Epub 2022 Jun 27. Gut. 2022. PMID: 35760493 Free PMC article. Clinical Trial.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Racecadotril for acute diarrhoea in children.Cochrane Database Syst Rev. 2019 Dec 19;12(12):CD009359. doi: 10.1002/14651858.CD009359.pub2. Cochrane Database Syst Rev. 2019. PMID: 31858591 Free PMC article.
References
-
- WHO . Geneva: 2005. The treatment of diarrhoea.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
