Randomised feasibility study of an intestinal adsorbent in acute diarrhoea in The Gambia
- PMID: 39855680
- PMCID: PMC11759216
- DOI: 10.1136/bmjpo-2024-003133
Randomised feasibility study of an intestinal adsorbent in acute diarrhoea in The Gambia
Abstract
Background: Diarrhoea remains a leading cause of death in children. An intestinal adsorbent may reduce diarrhoea duration and severity.
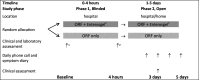
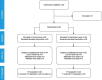
Methods: Randomised controlled feasibility trial with two phases: phase 1 (0-4 hours and double-blind) and phase 2 (up to 5 days and open-label). 50 children aged 6-59 months with acute diarrhoea presenting with no or some dehydration to the emergency paediatric unit and outpatient clinic at Edward Francis Small Teaching Hospital, Banjul, The Gambia were randomised to either standard treatment (oral rehydration fluid and zinc) or standard treatment with polymethylsiloxane polyhydrate for up to 5 days.
Results: Recruitment was completed in 7 months. All but one child completed the study. There were no major protocol deviations although patient-held diaries did not collect reliable information. Time from randomisation to the last watery stool (primary outcome) was shorter in the intervention than control arm (mean difference -19.3 hours, 95% CI -30.9 to -7.8). Stool frequency was lower in the intervention arm on days 2 (95% CI -0.8 to -1.3 to -0.3) and 3 (95% CI -0.8; -1.3 to -0.3). One serious event (death) occurred in the control arm.
Conclusions: A randomised, controlled trial is feasible. Further clinical trials are warranted to confirm the efficacy of polymethylsiloxane polyhydrate in acute diarrhoea and inform management guidelines.
Trial registration number: PACTR202302683128875.
Keywords: Child Health; Gastroenterology; Low and Middle Income Countries.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: CH is Research Director and EM is the Chief Executive Officer for Enteromed, UK the UK supplier of Enterosgel and study sponsor. RH is Chief Medical Officer for Bioline Products s.r.o., the manufacturer of Enterosgel. CH, EM and RH were not involved in undertaking any study procedures in The Gambia including data collection, analysis and interpretation. All other authors declare no potential conflicts of interest.
Figures
References
-
- WHO . Geneva: 2005. The treatment of diarrhoea.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
