Specific types of male infertility are correlated with T cell exhaustion or senescence signatures
- PMID: 39856063
- PMCID: PMC11759947
- DOI: 10.1038/s41467-025-56193-2
Specific types of male infertility are correlated with T cell exhaustion or senescence signatures
Abstract
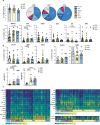
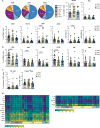
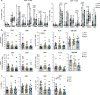
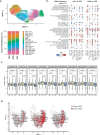
The association between male infertility and health status has yet to be unraveled. Here, by combining multiparameter phenotyping and scRNA-seq, we delineate the immune status of infertile men both at the semen and systemic levels. We first observe that young infertile men have a pro-inflammatory milieu with increased frequency of myeloid cells and inflammatory mediators in the seminal fluid and the peripheral blood, which are immune alterations typically observed in healthy elderly men. Transcriptomic profiling confirms the upregulation of genes associated with the interferon-gamma and -alpha responses in peripheral blood T cells of infertile men with oligo-astheno-teratozoospermia or non-obstructive azoospermia, with distinct T cell signatures of exhaustion and senescence discriminating the two infertile conditions. These findings provide evidence that subtypes of male infertility are characterized by specific immune signatures and unravel the potential link between infertility and the risk of developing secondary diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

