V3+ extends the V3 framework to ensure user-centricity and scalability of sensor-based digital health technologies
- PMID: 39856145
- PMCID: PMC11760348
- DOI: 10.1038/s41746-024-01322-2
V3+ extends the V3 framework to ensure user-centricity and scalability of sensor-based digital health technologies
Abstract
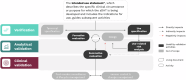
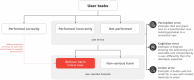
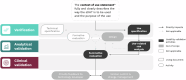
We propose the addition of usability validation to the extended V3 framework, now "V3+", and describe a pragmatic approach to ensuring that sensor-based digital health technologies can be used optimally at scale by diverse users. Alongside the original V3 components (verification; analytical validation; clinical validation), usability validation will ensure user-centricity of digital measurement tools, paving the way for more inclusive, reliable, and trustworthy digital measures within clinical research and clinical care.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: J.P.B. is a former employee of Philips and Signifier Medical Technologies and holds company stock or stock options, and reports consulting income from Apnimed and Koneksa Health. R.B. is an employee of Regeneron Pharmaceuticals Inc. and holds company stock or stock options. B.C. is an employee of Genentech and holds company stock. C.C. is an employee of Stel Life Inc. C.C.G. is an employee of ActiGraph LLC. B.H. is a former employee of Johnson & Johnson Innovative Medicine and holds company stock or stock options. N.H.M. is an employee of Exponent Inc. and holds company stock or stock options. E.S.I. is an employee of Koneksa health and may own company stock. N.V.M. is an employee of Johnson & Johnson Innovative Medicine and holds company stocks or stock options. S.Mo. is an employee of Sysnav Healthcare. O.P. is an employee of AARDEX Group. E.S. is a member of the NPJ Digital Medicine editorial board. T.S. is an employee of Genentech and owner of shares of Roche stock. A.T. is a paid consultant for Synergen Health Technologies; Siemens Healthineers; Gabi Smartcare; and Medtronic (relationship is concluded). S.V. is an employee of Johnson & Johnson Innovative Medicine and holds company stock or stock options. B.Va. is a former employee of Byteflies and holds company stock or stock options. BVr is an employee of AARDEX Group and holds company stock or stock options. The remaining authors declare no competing interests: J.C., S.Mc., S.P., M.S., W.vd.B., J.C.G.
Figures


References
-
- Taguibao, C. Celebrating the fourth anniversary of the Library of Digital Endpoints! Digital Medicine Society (DiMe)https://dimesociety.org/celebrating-the-fourth-anniversary-of-the-librar... (2023).
-
- Bellerophon. A study to assess pulsed inhaled nitric oxide in subjects with pulmonary fibrosis at risk for pulmonary hypertension (REBUILD). Clinical Trials https://clinicaltrials.gov/study/NCT03267108 (2023).
-
- Servais, L. et al. First regulatory qualification of a digital primary endpoint to measure treatment efficacy in DMD. Nat. Med.29, 2391–2392 (2023). - PubMed
-
- U.S. Food and Drug Administration. Medical Device Development Tool (MDDT) summary of evidence and basis of qualification Apple atrial fibrillation history feature. U.S. Food and Drug Administration https://www.fda.gov/media/178230/download (2024).
Publication types
LinkOut - more resources
Full Text Sources

