Digoxin for reduction of circulating tumor cell cluster size in metastatic breast cancer: a proof-of-concept trial
- PMID: 39856336
- PMCID: PMC12003195
- DOI: 10.1038/s41591-024-03486-6
Digoxin for reduction of circulating tumor cell cluster size in metastatic breast cancer: a proof-of-concept trial
Abstract
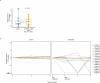
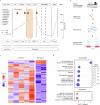
The presence of circulating tumor cell (CTC) clusters is associated with disease progression and reduced survival in a variety of cancer types. In breast cancer, preclinical studies showed that inhibitors of the Na+/K+ ATPase suppress CTC clusters and block metastasis. Here we conducted a prospective, open-label, proof-of-concept study in women with metastatic breast cancer, where the primary objective was to determine whether treatment with the Na+/K+ ATPase inhibitor digoxin could reduce mean CTC cluster size. An analysis of nine patients treated daily with a maintenance digoxin dose (0.7-1.4 ng ml-1 serum level) revealed a mean cluster size reduction of -2.2 cells per cluster upon treatment (P = 0.003), meeting the primary endpoint of the study. Mechanistically, transcriptome profiling of CTCs highlighted downregulation of cell-cell adhesion and cell-cycle-related genes upon treatment with digoxin, in line with its cluster-dissolution activity. No treatment-related adverse events occurred. Thus, our data provide a first-in-human proof of principle that digoxin treatment leads to a partial CTC cluster dissolution, encouraging larger follow-up studies with refined Na+/K+ ATPase inhibitors and that include clinical outcome endpoints. ClinicalTrials.gov identifier: NCT03928210 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: N.A. is a co-founder and member of the board of PAGE Therapeutics AG, Switzerland, listed as an inventor in patent applications related to CTCs, a paid consultant for companies with an interest in liquid biopsies, and a Novartis shareholder. C.R. is a co-founder of PAGE Therapeutics AG, Switzerland. The other authors declare no competing interests.
Figures





References
-
- GLOBOCAN 2020: new global cancer data. UICChttps://www.uicc.org/news/globocan-2020-new-global-cancer-data (2020).
-
- Gennari, A. et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol.32, 1475–1495 (2021). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

