Sex chromosomes and sex hormones differently shape microglial properties during normal physiological conditions in the adult mouse hippocampus
- PMID: 39856696
- PMCID: PMC11762133
- DOI: 10.1186/s12974-025-03341-6
Sex chromosomes and sex hormones differently shape microglial properties during normal physiological conditions in the adult mouse hippocampus
Abstract
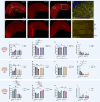
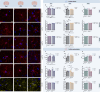
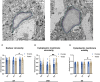
The brain presents various structural and functional sex differences, for which multiple factors are attributed: genetic, epigenetic, metabolic, and hormonal. While biological sex is determined by both sex chromosomes and sex hormones, little is known about how these two factors interact to establish this dimorphism. Sex differences in the brain also affect its resident immune cells, microglia, which actively survey the brain parenchyma and interact with sex hormones throughout life. However, microglial differences in density and distribution, morphology and ultrastructural patterns in physiological conditions during adulthood are largely unknown. Here, we investigated these aforementioned properties of microglia using the Four Core Genotypes (FCG) model, which allows for an independent assessment of gonadal hormones and sex chromosomal effects in four conditions: FCG XX and Tg XY- (both ovaries); Tg XXSry and Tg XYSry (both testes). We also compared the FCG results with XX and XY wild-type (WT) mice. In adult mice, we focused our investigation on the ventral hippocampus across different layers: CA1 stratum radiatum (Rad) and CA1 stratum lacunosum-moleculare (LMol), as well as the dentate gyrus polymorphic layer (PoDG). Double immunostaining for Iba1 and TMEM119 revealed that microglial density is influenced by both sex chromosomes and sex hormones. We show in the Rad and LMol that microglia are denser in FCG XX compared to Tg XYSry mice, however, microglia were densest in WT XX mice. In the PoDG, ovarian animals had increased microglial density compared to testes animals. Additionally, microglial morphology was modulated by a complex interaction between hormones and chromosomes, affecting both their cellular soma and arborization across the hippocampal layers. Moreover, ultrastructural analysis showed that microglia in WT animals make overall more contacts with pre- and post-synaptic elements than in FCG animals. Lastly, microglial markers of cellular stress, including mitochondrion elongation, and dilation of the endoplasmic reticulum and Golgi apparatus, were mostly chromosomally driven. Overall, we characterized different aspects of microglial properties during normal physiological conditions that were found to be shaped by sex chromosomes and sex hormones, shading more light onto how sex differences affect the brain immunity at steady-state.
Keywords: Density; Distribution; Four core genotypes; Hippocampus; Microglia; Morphology; Mouse; Scanning Electron Microscopy; Sex differences; Ultrastructure.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: All animal experiments and procedures were approved by the Université Laval (2021-830 and 2023-1372) and the University of Victoria (AUP 2020-013) animal care committees, strictly following the recommendations from the Canadian Council on Animal Care. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

